
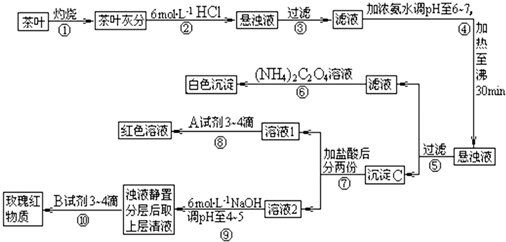
��14�֣������ҹ�����ϲ������Ʒ��ijУ��ѧ��ȤС���ͬѧ�������ʵ�������Լ����Ҷ�к���Ca��A1��Fe����Ԫ�ء�
�����ϲ�ѯ���������[(NH4)2C2O4]����������ʡ�����ƣ�CaC2O4��������ˮ��Ca2+��
A13+��Fe3+��ȫ������pH��Ca(OH)2��pH��13��A1(OH)3��pH��5.5��Fe(OH)3��pH��4.1��
�Ը����������̼���Ϣ��գ�
��1������ڼ������������ ��
��2������۲������õ��IJ��������� ��
��3��д��������м���Ca2+���ڵ����ӷ���ʽ ��
��4��д������C������Ҫ���ʵĻ�ѧʽ ��
��5��д���������ѡ��A�Լ��Ļ�ѧʽ ��
��6�������������� ��
�²ⲽ����Ŀ���� ��
��14�֣�
��1��ʹCa2+��Al3+��Fe3+��������ʹCa2+��Al3+��Fe3+�ܽ⣩��2�֣�ֻ�ش��1����2����
�ӣ���1�֣�����ʹ�������ܽ��γ���Һ�Ȳ�����𰸸�1�֣�
��2����ͨ©�����ձ��������� ��2�֣�
��3��Ca2++(NH4)2C2O4= CaC2O4��+2NH4+ ��2�֣�
��4��Fe(OH)3��Al(OH)3 �� ��1�֣���2�֣�
��5��KSCN ��NH4SCN ��2�֣�
��6��ʹFe3+ת��ΪFe(OH)3���� ��2�֣��� ����Al3+������Ԫ�أ����� ��2�֣�
����:


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
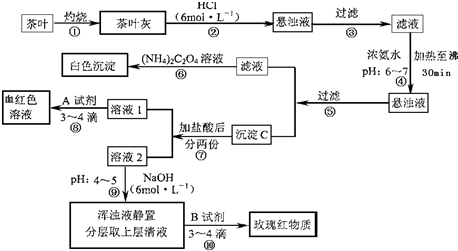
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����IJ������ϡ�����李�(NH4)2C2O4������������ʡ������(CaC2O4)������ˮ��Ca2+��Al3+��Fe3+��ȫ������pH��Ca(OH)2��pH��13��Al(OH)3��pH��5.5��Fe(OH)3��pH��4.1��

�Ը����������̼���Ϣ��գ�
(1)����ڼ������������____________________________________________________��
(2)д������Ca2+�����ӷ���ʽ________________________________________________��
(3)д������C������Ҫ���ʵĻ�ѧʽ____________��
(4)д���������A�Լ����ɺ�ɫ��Һ�����ӷ���ʽ______________________________��
(5)������������_________________���²ⲽ����Ŀ����______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com