
CH3OH��g��+H2O��g��![]() CO2��g��+3H2��g�����÷�Ӧ������ӦΪ���ȷ�Ӧ���������ĿҪ��ش��������⣺
CO2��g��+3H2��g�����÷�Ӧ������ӦΪ���ȷ�Ӧ���������ĿҪ��ش��������⣺
��1���������������������½����¶ȣ����淴Ӧ������ �������С�����䡱����ͬ�����������������������¼�ѹ��������Ӧ������ ��
��2��һ�������£������Ϊ2L���ܱ������г���1molCH3OH(g)��3molH2O(g)��20s ��û�������ѹǿ�Ƿ�Ӧǰ��1.2�������ü״���ʾ�÷�Ӧ������Ϊ ��
��3���жϸÿ��淴Ӧ�ﵽƽ��״̬�������ǣ�����ţ����� ��
�� A v����CH3OH��=v��(CO2)
B ���������ܶȲ���
C ��������ƽ����Է�����������
D CH3OH��H2O��CO2��H2��Ũ�ȶ����ٷ����仯
��4����һ�������£����÷�Ӧ���ڻ�ѧƽ��״̬ʱ�����в�����ʹ��ѧƽ�����淴Ӧ�� ���ƶ������� ������ţ���
A�������¶ȡ��� B�������¶ȡ��� C������ѹǿ
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
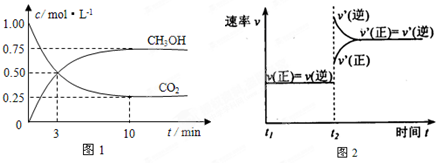
| ���� | CH3OH | CH3OCH3 | H2O |
| c/mol?L-1 | 0.44 | 0.60 | 0.60 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
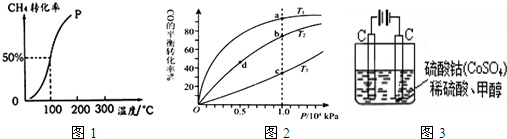
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
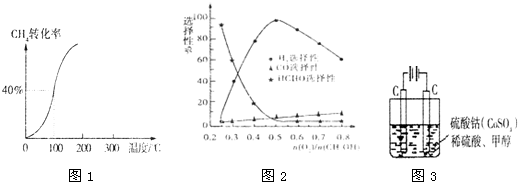
| 1 |
| 2 |
| c(H2) |
| c(CH3OH) |
| ���� |
| ���� |
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
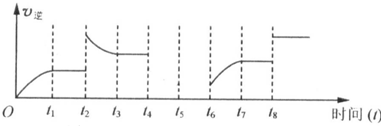
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
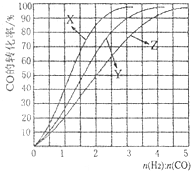 ��ҵ�ϳ�����CO��H2�ϳɿ�������Դ�״���
��ҵ�ϳ�����CO��H2�ϳɿ�������Դ�״����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com