
| �ζ����� | ��Ʒ������/g | ϡ��������/mL | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 0.3000 | 1.02 | 21.03 |
| 2 | 0.3000 | 2.00 | 21.99 |
| 3 | 0.3000 | 0.20 | 20.20 |

���� ��1��Na2CO3��NaHCO3�������̼���������ȷֽ�����̼���ơ�������̼��ˮ��
��2�����ݼ��ȷ�Ӧ�����з�Ӧǰ�������仯����̼�����Ƶõ�̼������������̼��������������
��3��������Һϡ�����������Ȼ����غ�ɼ������������������ʱ���ӿ̶��ߣ����������Ƶ���Һ�����ƫС���ݴ��жϣ�
��4���ζ�ʵ�����÷�ָ̪ʾ�յ㣬�����ķ�ӦΪ��H++CO32-=HCO3-�����ݷ�Ӧ������ϵ����õ�̼�������ʵ���������õ�̼��������������
��5���ڷ�Һ©���м�������ˮ����ͼ���Ӻ�װ�ã��ر�ֹˮ��a����ֹˮ�м�ס��Ƥ��c������b����װ�ò�©�������ڷ�Һ©������������ѹǿС�������Һ�岻����ȫ���£�����c���ӷ�Һ©����������ƿ��ʹ��������ѹǿ��ȣ�ͬʱ��СҺ�������������������
��6��Ϊ����߲ⶨ��ȷ�ԣ������������Ӧѡ�������ܸ�ȷ��
��� �⣺��1��Na2CO3��NaHCO3�������̼���������ȷֽ�����̼���ơ�������̼��ˮ�������з�����Ӧ�Ļ�ѧ����ʽΪ��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O+CO2����
�ʴ�Ϊ��2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O+CO2����
��2���ٳ���������������ΪA g���ڳ���װ����������������ΪBg���ۼ��ȣ�����ȴ���ݳ��������Ͳ����������ΪC g�����ظ������ݲ�����ֱ�����أ�����ΪDg�����ݼ��㷽����������Ҫ����ֵΪ���ٳ���������������ΪA g���ڳ���װ����������������ΪBg���ۼ��ȣ�����ȴ�����������صõ���Ӧ�������������Ӧǰ��������Ϊ��B-A-D������Ϸ�Ӧ2NaHCO3$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+H2O+CO2����ǰ��������������������̼�����Ƶ�������̼��������ΪB-A-̼����������������õ�̼������������������������Ҫ��ֵΪA��B��D��
�ʴ�Ϊ��A��B��D��
��3��������Һϡ�����������Ȼ����غ�ɼ������������=$\frac{0.100mol/L��0.1L}{2.0mol/L}$=5.00mL������ʱ���ӿ̶��ߣ����������Ƶ���Һ�����ƫС�����Իᵼ����Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ��5.00��ƫ�ߣ�
��4��ͼ����������Һ���ƽ��Ϊ��$\frac{21.03-1.2+21.99-2.00+20.20-0.20}{3}$ml=20.00ml
���ݷ�Ӧ H++CO32-=HCO3-��
0.020L��0.100mol/L 0.002mol
m��Na2CO3��=0.002mol��106g/mol=0.212g
̼������������=$\frac{0.212g}{0.3g}$��100%=70.7%��
�ʴ�Ϊ��70.7%��
��5���ڷ�Һ©���м�������ˮ����ͼ���Ӻ�װ�ã��ر�ֹˮ��a����ֹˮ�м�ס��Ƥ��c������b����װ�ò�©�������ڷ�Һ©������������ѹǿС�������Һ�岻����ȫ���£���������ˮ����һ��ʱ������������ҳ���һ��ʱ�䣬�ڷ�Һ©���м�������ˮ����ͼ���Ӻ�װ�ã��ر�ֹˮ��a����ֹˮ�м�ס��Ƥ��c������b������Һ©���е�ˮ���������²����ж�װ�������ԣ������Ƿ�Һ©���Ͽڰ���δ��Ӧ���������İ��ۣ�����c���ӷ�Һ©����������ƿ��ʹ��������ѹǿ��ȣ����ڷ�Һ©���е�Һ�����£�ͬʱ��СҺ�������������������
�ʴ�Ϊ����ȷ����ƽ����ѹ��������Һ����������СҺ�������������������
��6��Ϊ����߲ⶨ��ȷ�ԣ������������Ӧѡ�������ܸ�ȷ��Ӧѡ��ACװ�ý��вⶨ��
�ʴ�Ϊ��C��
���� ���⿼����������ɵ� ʵ��̽����ʵ��ⶨ���������жϣ���Ҫ��ʵ������������������ʵ�����Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ۢߢ� | B�� | �ڢۢߢ� | C�� | �ۢܢݢ� | D�� | �ܢݢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2Na+2H2O�T2NaOH+H2�� | |
| B�� | 2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2�� | |
| C�� | 2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+H2��+Cl2�� | |
| D�� | 2Na2O2+2H2O�T4NaOH+O2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͭƬ��Ӧ | B�� | ��ʯ����Һ��Ӧ | ||
| C�� | �ò�������պ����Ϳ��ֽ�� | D�� | ����пƬ���Ƿ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
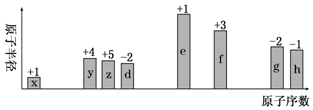
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 Ϊ�˲ⶨ�����³´�л����������CO2�����������ijѧ������С���������ͼ��ʾ��ʵ��װ�ã�ʵ�����ù���NaOH��Һ���������е�CO2��ȷ����ƿ������Һ����CO2������ؼ�ʣ������������ʵ��ʱֻ���������ͺ�����������գ�
Ϊ�˲ⶨ�����³´�л����������CO2�����������ijѧ������С���������ͼ��ʾ��ʵ��װ�ã�ʵ�����ù���NaOH��Һ���������е�CO2��ȷ����ƿ������Һ����CO2������ؼ�ʣ������������ʵ��ʱֻ���������ͺ�����������գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | a���Ӧ��NH3•H2O��Һ��pH=12 | |
| B�� | b������Һ�У�c��C1-��=c��NH4+��+c��NH3•H2O�� | |
| C�� | c������Һ�У�c��H+����[��NH3•H2O��] | |
| D�� | d������Һ�У�c��Cl-����c��NH4+����c��H+����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | S������ɫ�� | B�� | NO����ɫ�� | C�� | ��ˮ����ͭ����ɫ�� | D�� | NO2������ɫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 222 | B�� | 429.5 | C�� | 462 | D�� | 924 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com