
��֪�Ҵ����Ժ���ˮCaCl2��Ӧ��������ˮ��CaCl2??6C2H5OH ��
�й��Լ��ķе����£�CH3COOC2H5 77.1�棻 C2H5OC2H5 34.5�棻
C2H5OH 78.3�棻 CH3COOH 118�档
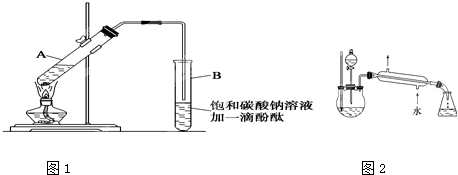
ij����С��������ͼ��ʾװ���Ʊ����������ֲ�Ʒ���Իش��������⣺
��1��������ƿ�м�����Լ��ǣ� �����ᡢ�ƾ���Ũ���ᣬ
����ŨH2SO4�������� �� ��
��2������������������������� �� ��ȥ�ͷе����֡��ռ��е���76��~120��
֮�����֣������㵹�뱥��Na2CO3��Һ�й۲쵽�������ǣ� ��
����ʵ���б���̼������Һ��������_____������ţ�
A���к�������Ҵ��� B���к����Ტ�ܽ��Ҵ���
C�������������ɣ�������IJ��� D�������ڷֲ�������
��3��д������ʵ������CH3COOH��CH3CH218OH��Ũ�����Ʊ����������Ļ�ѧ����ʽ��
��
��4��������ֲ�Ʒ�����������ټ�����ˮCaCl2�������Ŀ���ǣ�
Ϊ�˸�������������ѡ�õ���Ѹ����Ϊ ________ ������ţ�
A������������ B����ˮ������ C����ʯ�� D��������������
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g/cm3�� |
| �ҡ��� | -117.0 | 78.0 | 0.79 |
| �ҡ��� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���ᣨ98%�� | - | 338.0 | 1.84 |
 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�Ҵ����Ժ���ˮCaCl2��Ӧ��������ˮ��CaCl2•6C2H5OH ��
�й��Լ��ķе����£�CH3COOC2H5 77.1�棻 C2H5OC2H5 34.5�棻
C2H5OH 78.3�棻 CH3COOH 118�档
ij����С��������ͼ��ʾװ���Ʊ����������ֲ�Ʒ���Իش��������⣺
��1��������ƿ�м�����Լ��ǣ� �����ᡢ�ƾ���Ũ���ᣬ
����ŨH2SO4�������� �� ��
��2������������������������� �� ��ȥ�ͷе����֡��ռ��е���76��~120��
֮�����֣������㵹�뱥��Na2CO3��Һ�й۲쵽�������ǣ� ��
����ʵ���б���̼������Һ��������_____������ţ�
A���к�������Ҵ��� B���к����Ტ�ܽ��Ҵ���
C�������������ɣ�������IJ��� D�������ڷֲ�������
��3��д������ʵ������CH3COOH��CH3CH218OH��Ũ�����Ʊ����������Ļ�ѧ����ʽ��
��
��4��������ֲ�Ʒ�����������ټ�����ˮCaCl2�������Ŀ���ǣ�
Ϊ�˸�������������ѡ�õ���Ѹ����Ϊ ________ ������ţ�
A������������ B����ˮ������ C����ʯ�� D��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ϻ������ָ���5�¸߶���ѧ���� ���ͣ�ʵ����
��֪�Ҵ����Ժ���ˮCaCl2��Ӧ��������ˮ��CaCl2?6C2H5OH ��
�й��Լ��ķе����£�CH3COOC2H5 77.1�棻 C2H5OC2H5 34.5�棻
C2H5OH 78.3�棻 CH3COOH 118�档
ij����С��������ͼ��ʾװ���Ʊ����������ֲ�Ʒ���Իش��������⣺
��1��������ƿ�м�����Լ��ǣ������ᡢ�ƾ���Ũ���ᣬ
����ŨH2SO4�������� �� ��
��2������������������������� �� ��ȥ�ͷе����֡��ռ��е���76��~120��
֮�����֣������㵹�뱥��Na2CO3��Һ�й۲쵽�������ǣ� ��
����ʵ���б���̼������Һ��������_____������ţ�
A���к�������Ҵ��� B���к����Ტ�ܽ��Ҵ���
C�������������ɣ�������IJ��� D�������ڷֲ�������
��3��д������ʵ������CH3COOH��CH3CH218OH��Ũ�����Ʊ����������Ļ�ѧ����ʽ��
��
��4��������ֲ�Ʒ�����������ټ�����ˮCaCl2�������Ŀ���ǣ�
Ϊ�˸�������������ѡ�õ���Ѹ����Ϊ ________ ������ţ�
A������������ B����ˮ������ C����ʯ�� D��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ϻ���5�¸߶���ѧ���� ���ͣ�ʵ����
��֪�Ҵ����Ժ���ˮCaCl2��Ӧ��������ˮ��CaCl2•6C2H5OH ��
�й��Լ��ķе����£�CH3COOC2H5 77.1�棻 C2H5OC2H5 34.5�棻
C2H5OH 78.3�棻 CH3COOH 118�档
ij����С��������ͼ��ʾװ���Ʊ����������ֲ�Ʒ���Իش��������⣺
��1��������ƿ�м�����Լ��ǣ� �����ᡢ�ƾ���Ũ���ᣬ
����ŨH2SO4�������� �� ��
��2������������������������� �� ��ȥ�ͷе����֡��ռ��е���76��~120��
֮�����֣������㵹�뱥��Na2CO3��Һ�й۲쵽�������ǣ� ��
����ʵ���б���̼������Һ��������_____������ţ�
A���к�������Ҵ��� B���к����Ტ�ܽ��Ҵ���
C�������������ɣ�������IJ��� D�������ڷֲ�������
��3��д������ʵ������CH3COOH��CH3CH218OH��Ũ�����Ʊ����������Ļ�ѧ����ʽ��
��
��4��������ֲ�Ʒ�����������ټ�����ˮCaCl2�������Ŀ���ǣ�
Ϊ�˸�������������ѡ�õ���Ѹ����Ϊ ________ ������ţ�
A������������ B����ˮ������ C����ʯ�� D��������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com