
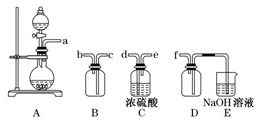
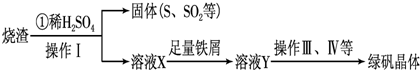
ʵ���������᳧��������Ҫ�ɷ�ΪFe2O3������FeS��SiO2�ȣ��Ʊ���������ʽ�������ľۺ�����̷�(FeSO4•7H2O)���йصĹ����������£�

��1�������̢��в���������ͨ��������Һ�У���Һ������ɫ����___________�����ţ���
A��Ʒ����Һ B����ɫʯ����Һ
C������KMnO4��Һ D����ˮ
��2���ڢ�����֪FeS����Ԫ������Fe3+��SԪ�����ɵ�����FeS��O2��H2SO4��Ӧ�ķ���ʽ�� ��
��3���ڢ��У�������������___________________________��
��4���ڢ��У�����Ũ����Ҫ�Ĺ������������ƾ����⣬����_______________________��
��5���ڢ��У�����ҺZ���Ƶ�70��80���Ŀ����________________________________��
��6��Ϊ����������Ʒ����Ԫ�ص�������������������ʵ�飨���������в�����Ԫ�غ���Ԫ�أ�����ȡ2.700g��Ʒ������Ʒ��������������μӹ�����BaCl2�����ˡ�ϴ�ӡ�����������������Ϊ3.495g�����þ�����Ҫ�ɷ�Ϊ[Fe(OH)(SO4)]n����þ�����Ʒ����Ԫ�ص���������[Mr (BaSO4) =233��Mr (Fe) =56]����д��������̣��������4λ��Ч���֣���
��16�֣���1��B��2�֣�
��2��4FeS+3O2+6H2SO4=2Fe2(SO4)3+ 6H2O +4S��3�֣�д���ӷ���ʽ��4FeS+3O2+12H+=4Fe3++6H2O+4S��Ҳ�÷֣�
��3��Fe�������ۣ���2�֣�
��4���������ձ������ʯ�������۷֣�����������2�֣�
��5�������¶ȴٽ�Fe3+��ˮ�⣨2�֣��������¶Ȳ����ھ������γɣ�������ȫˮ���ˣ���1�֣� ע�����¶�̫�Ͳ�����ˮ�⣬�¶ȹ����������ɾ���������˼�������Ҳ�÷�
��6����4�֣�����Ԫ�ص�����Ϊxg������������Ԫ���غ�ɵù�ϵʽ����
n Fe ��[Fe(OH)(SO4)]n �� nBaSO4 (1��)
56n 233n
x 3.495g
56n/233n=x/3.495g��1�֣�
x=0.84g��1�֣�
����Ʒ����Ԫ�ص���������=0.84/2.700��100%=31.11%��1�֣�
��������
�����������1��SiO2�������ȶ�������ʱ���ܷ�Ӧ����S����ʱ����O2��Ӧ����SO2���壻�����������Ư���ԣ���ʹƷ����Һ��ɫ����A������������������������ͨ�ԣ�������ˮ����ˮ��Ӧ���������ᣬ����Һ�����ԣ���ʹ��ɫʯ����Һ��죬���Dz�����ɫ����B��ȷ������������л�ԭ�ԣ����Ը�����ؾ���ǿ�����ԣ���������������ԭ��Ӧ�������ʹ���Ը��������Һ��ɫ����C������ˮ���������ԣ��ܱ���������ԭΪ�����ӣ����SO2��ʹ��ˮ��ɫ����D����2�����ݻ��ϼ�����������Σ�FeS�ǻ�ԭ����O2�������������ݵ��ӵ�ʧ����ɡ�ԭ���غ�ԭ���ɵã�4FeS+3O2+6H2SO4=2Fe2(SO4)3+ 6H2O +4S��4FeS+3O2+12H+=4Fe3++6H2O+4S����3���̷�Ϊ��ˮ�������������ɴ��ƶϲ���۵�Ŀ�Ľ�Fe3+��ԭΪFe2+�����ݳ��Ӳ����������ʵ�ԭ��Ӧ�ü����������м�����ۣ���4������Ũ������ȴ�ᾧ��Ҫʹ�õ��������������ձ������������ƾ��ƣ���5��������ˮ�⣬ˮ�ⷴӦ�����ȷ�Ӧ���¶ȹ��Ͳ�����Fe3+��ˮ�⣬�����¶ȿ��Դٽ�Fe3+��ˮ�⣬�����¶ȹ���ʱFe3+�ܳ���ˮ���Ϊ����������Ҳ��۳�Ϊ��������˲���70��80�����������6������Ԫ�ص�����Ϊxg������������Ԫ���غ�ɵù�ϵʽ����
n Fe ��[Fe(OH)(SO4)]n �� nBaSO4
56n 233n
x 3.495g
����ʽ��֮�ȵ�������֮�ȣ���56n/233n=x/3.495g����ã�x=0.84g��������Ʒ����Ԫ�ص���������=0.84/2.700��100%=31.11%��
���㣺���������Ʊ��Ĺ������̴��⣬�漰���������������Ҫ���ʡ�������ԭ��Ӧ����ʽ����ƽ�������ķ������ᴿ����ѧʵ����������������ˮ�⡢�������Ҫ���ʡ�������ɵIJⶨ�ͼ��㡣


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� |
| �� |
| c2(SO3) |
| c(O2)?c2(SO2) |
| c2(SO3) |
| c(O2)?c2(SO2) |
| O | - 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

- 4 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com