
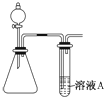
 ��������һ����̼������������������Ƿdz���Ҫ�ķǽ���������о���Щ������Ի������������⻯ѧ��Ӧԭ����������Ҫ���壮
��������һ����̼������������������Ƿdz���Ҫ�ķǽ���������о���Щ������Ի������������⻯ѧ��Ӧԭ����������Ҫ���壮���� ��1������������һ�����������ͨ��ˮ���ܱ�ˮ��ȫ�����������ᣬ��Ӧ����ʽΪ��4NO2+O2+2H2O�T4HNO3�����ݻ��ϼ������͵���ת�ƽ�����ؼ��㣻
��2��A������β�����������ã�CO+NO$\frac{\underline{����}}{��}$NO2+C�����ɶ��������ж������壻
B��ʯ����Ca��OH��2�����������������֮����Է�����Ӧ����������ƺ�ˮ��
C��ȼ�շ���ȥβ�������⣺2H2S+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2SO2+2H2O�������ж������������
D��������һ��������һ�������·�Ӧ�������ǵ�����ˮ��
��3��Ӱ������������������Ʒ�Ӧ���������������������Դ�С����A��̼����������ȼ�տ�������CO����CO2��������������ͬ�����ﲻͬ��
B����������������������������ȼ�ղ���Ϊ���������д�����ת��Ϊ��������
C�����������ڵ�ȼ�����������������ƣ����������������������ƣ������������йأ�
D��ͭ��������Һ��Ӧ���������Ũ���йأ�Ũ�ȴ����ɶ���������Ũ��С����һ��������
��2NO2+2NaOH�TNaNO3+NaNO2+H2O��NO+NO2+2NaOH�T2NaNO2+H2O��NO����������������Һ��ˮ��֪�������������Թ���������$\frac{VN{O}_{2}}{VNO}$��1��
��� �⣺��1������������һ�����������ͨ��ˮ���ܱ�ˮ��ȫ�����������ᣬ��Ӧ����ʽΪ��4NO2+O2+2H2O�T4HNO3����Ӧ��4NA������ת�ƣ���μӷ�Ӧ�Ķ������������ʵ���Ϊ4mol�����Ը÷�Ӧ��a��NA������ת�ƣ���μӷ�Ӧ�Ķ������������ʵ���Ϊa mol��
�ʴ�Ϊ��4NO2+O2+2H2O�T4HNO3��a mol��
��2��A������β�����������ã�CO+NO$\frac{\underline{����}}{��}$NO2+C�����ɶ��������ж������壬��A����
B��ʯ����Ca��OH��2�����������������֮����Է�����Ӧ����������ƺ�ˮ����SO2+Ca��OH��2�TCaSO3+H2O����B��ȷ��
C��ȼ�շ���ȥβ�������⣺2H2S+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2SO2+2H2O�������ж������������C����
D��������һ��������һ�������·�Ӧ�������ǵ�����ˮ����4NH3+6NO$\frac{\underline{MnO_2}}{��}$5N2+6H2O����D��ȷ��
��ѡAC��
��3����A��̼����������ȼ�տ�������CO����CO2����������Դ�С�йأ���A���ϣ�
B����������������������������ȼ�ղ���Ϊ���������д�����ת��Ϊ���������������йأ���B�����ϣ�
C�����������ڵ�ȼ�����������������ƣ����������������������ƣ������������йأ���C�����ϣ�
D��ͭ��������Һ��Ӧ���������Ũ���йأ�Ũ�ȴ����ɶ���������Ũ��С����һ����������D�����ϣ�
��ѡA��
��2NO2+2NaOH�TNaNO3+NaNO2+H2O��NO+NO2+2NaOH�T2NaNO2+H2O��NO����������������Һ��ˮ��֪�������������Թ���������$\frac{VN{O}_{2}}{VNO}$��1����ѡ��C��
���� �����漰������ԭ��Ӧ�е����غ�ļ����Լ���ȥ������Ⱦ��Ŀ����ԣ������ۺ�֪ʶ�Ŀ����⣬�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| A | ԭ�Ӱ뾶��С |
| B | ����3���ܼ��ϵĵ�������� |
| C | ���⻯��ķе��ͬ����������Ԫ���⻯��ķе�� |
| D | 2p����ϳɶԵ�������δ�ɶԵ�������� |
| E | N�������Ϊ1���ڲ���ȫ���������� |
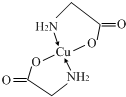 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����к���̼������ʴ�����ȴ����� | |
| B�� | �������ӵ��������������Ӵ������� | |
| C�� | ˮ��ĸ�բ������Դ�������ױ���ʴ | |
| D�� | �ִ�������Ƕп�飬���岻�ױ���ʴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����;�����մﵽƽ��ʱ����ϵ�ڻ�������Ũ����ͬ | |
| B�� | ����;�����մﵽƽ��ʱ����ϵ�ڻ������İٷ������ͬ | |
| C�� | �ﵽƽ��ʱ����;���ķ�Ӧ���ʦ�1���ڢ�;���ķ�Ӧ���ʦ�2 | |
| D�� | �ﵽƽ��ʱ����;�����������ܶȵ��ڢ�;�����������ܶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ᡢ��ζ��ǵ���� | |
| B�� | ���ǡ��ƾ��Ƿǵ���� | |
| C�� | ������ǿ����� | |
| D�� | NH3��ˮ��Һ�ܵ��磬��NH3���ǵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 110 kJ | B�� | 440 kJ | C�� | 1100 kJ | D�� | 2216 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2��1 | B�� | 1��1 | C�� | 2��3 | D�� | 1��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com