
��������Խ��Խ�ܵ����ǵĹ�ע���±�����ʡ���ֳ��п��������ܱ���
�������������������������������� ��Ⱦָ���������������������� ��Ҫ��Ⱦ������������������ ���������ȼ�
�������������������������������� 98���������������������������� SO2���������������������������� ��
�ൺ���������������������������� 47���������������������������������������������������������������� ��
�Ͳ����������������������������� 103���������������������������� TSP���������������������������� ��
������������������������������ 90���������������������������� NOx���������������������������� ��
ע��TSP�������������NOx����������
��1�����ϱ���֪�������׳�������ij�����________��
��2�������������׳�������������е�SO2��O2�Ӵ�ʱ��SO2�Ჿ��ת��ΪSO3�����������������������________��������ţ�
A������������������������������������������������������������ B����ԭ��
C���������������������������������������������������������� D��������
��3������������Ҳ�Ǵ�����Ⱦ��֮һ��Ŀǰ������NO�ķ�������һ���������ð���һ��������ԭΪ������ˮ����ѧ�������룬����ͬ�����¿ɲ��ü۸��NH3�����˵���Ȼ��������NO���ɵõ�ͬ��Ч����д���÷�Ӧ�Ļ�ѧ����ʽ_________________________________________________________________________��
��4��ij�еȳ���ÿ��ȼúԼ3´106t���京������1.00%���㣬����ȫ��ת��ΪSO2��SO2��60.0%ת��Ϊ���ᣬ�൱�����ɶ��ٶ�98.0%�����
����ʽ���㣩
 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ߵ�ֱ��Ӱ��������������������Խ��Խ�ܵ����ǵĹ�ע������Ⱦ�Ŀ��������ʵijɷ��ж��֣����м��롶���������ձ���������Ⱦָ������Ŀ��SO2��CO��NO2��O3�Ϳ����������ȡ�
��ش��������⣺
�� S��N��O�ĵ�һ�������ɴ�С��˳��Ϊ ��
�� Ѫ�쵰���к���Fe2����CO����Ѫ�쵰��ϳ��ȶ���������ʹ���ж���
 �� д���������ӵĻ�̬�����Ų�ʽ ��
�� д���������ӵĻ�̬�����Ų�ʽ ��
�� CO�ж��ֵȵ����壬���г���������Ϊ ��
�� SO2��һ�ִ�����Ⱦ�Ϊ����SO2��Ⱦ���������糧�����г���
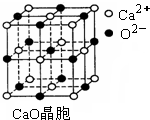
ȼú�м���CaO�ԡ����� CaO��������ͼ��ʾ������Ca2������λ
��Ϊ ��CaO�����NaCl�������������з�ʽ��ͬ���侧���ܷ�
��Ϊ��CaO��3 401kJ/mol��NaCl��786kJ/mol��CaO������۵� NaCl
������۵㣨����ڡ��������ڡ����ڡ�����
�� ��������������������ߣ���������Ļ�����ȫΪ���������ӣ����ڵĻ�����ȫ��ʳƷ��ȫҲԽ��
ԽΪ��������ע����ȩ��������Ҫ������Ⱦ��֮һ����е��ǣ�19.5 �棩���״��ǡ��پơ��е���Ҫ
�к����ʣ���е���64.65 �棩���״��ķе����Ը��ڼ�ȩ����Ҫԭ���ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
���������ߵ�ֱ��Ӱ��������������������Խ��Խ�ܵ����ǵĹ�ע������Ⱦ�Ŀ��������ʵijɷ��ж��֣����м��롶���������ձ���������Ⱦָ������Ŀ��SO2��CO��NO2��O3�Ϳ����������ȡ�
��ش��������⣺
�� S��N��O�ĵ�һ�������ɴ�С��˳��Ϊ ��
�� Ѫ�쵰���к���Fe2����CO����Ѫ�쵰��ϳ��ȶ���������ʹ���ж�![]()
�� д���������ӵĻ�̬�����Ų�ʽ ��
�� CO�ж��ֵȵ����壬���г���������Ϊ ��CO�ṹ�ЦҼ��ͧ�����Ŀ֮��Ϊ ![]()
��Fe(CO)5�����³�Һ̬���۵�Ϊ��20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe(CO)5�������� ___________ ��������ͣ���FeԪ�صĻ��ϼ� ��
�� SO2��һ�ִ�����Ⱦ�Ϊ����SO2��Ⱦ���������糧�����г���ȼú�м���CaO�ԡ����� CaO������ͼ��ʾ������Ca2����Χ����������λ��Ϊ ��CaO�����NaCl�������������з�ʽ��ͬ���侧���ֱܷ�Ϊ��CaO��3401kJ/mol��NaCl��786kJ/mol��CaO������۵� NaCl������۵㣨����ڡ��������ڡ����ڡ������������߾����ܲ������Ҫԭ���� ��

�� ��������������������ߣ���������Ļ�����ȫΪ���������ӣ����ڵĻ�����ȫ��ʳƷ��ȫҲԽ��ԽΪ��������ע����ȩ��������Ҫ������Ⱦ��֮һ����е��ǣ�19.5 �棩���״��ǡ��پơ��е���Ҫ�к����ʣ���е���64.65 �棩���״��ķе����Ը��ڼ�ȩ����Ҫԭ���ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008-2009ѧ��ɽ��ʡ�ൺ�и߶����£���ĩ��ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com