
��12�֣�ij��ɫ����Һ�п��ܴ��ڴ���Ag����Mg2����K����Cu2���е�һ�ֻ��֣�����д���пհס�
��1���������κ�ʵ��Ϳ��Կ϶���Һ�в����ڵ�������________________��
��2��ȡ����ԭ��Һ���������ϡ���ᣬ�а�ɫ�������ɣ��ټ������ϡ���ᣬ��������ʧ��˵��ԭ��Һ�п϶����ڵ�������________����Ӧ�����ӷ���ʽΪ_______________��
��3��ȡ��2������Һ�ӹ�����NaOH��Һ�����ְ�ɫ������˵��ԭ��Һ�п϶���________���йط�Ӧ�����ӷ���ʽΪ________________��
��4��ԭ��Һ�п��ܴ������ڵ���������
| A��Cl�� | B��NO3�� | C��CO3 2�� | D��OH�� |
����12�֣�
��1��Cu2����
��2��Ag+��Ag++Cl��=AgCl��
��3��Mg2+��Mg2++2OH��= Mg(OH)2��
��4��B ����ѡ���÷֣�
���������������1�������д���Cu2������Һ����ɫ������ɫ��Һ��һ��������Cu2������2����Cl����Ag+��Ӧ�����ɰ�ɫ��AgCl������������ˮ��ϡ���ᣬ��ʵ�飨2��˵��ԭ��Һ�п϶�����Ag+��ԭ����Ag++Cl��=AgCl������3��������ϡ���������ʵ�飨2��������Һ����Ag+��OH������H+��Ӧ������ˮ��OH������Mg2+��Ӧ�����ɰ�ɫ��Mg(OH)2��������ʵ�飨3��˵��ԭ��Һ�п϶���Mg2+���йط�ӦΪOH��+H+= H2O��Mg2++2OH��= Mg(OH)2������4����Cl����Ag+��Ӧ����ԭ��Һ�����ܺ��д�����Cl����A�����NO3����Ag+��Mg2+�����ܷ�Ӧ�������������ˮ���ɣ�B����ȷ��CO3 2����Ag+��Mg2+���ܷ�Ӧ�����ɰ�ɫ������C�����OH����Ag+��Mg2+���ܷ�Ӧ�����ɰ�ɫ������D�����
���㣺�������ӵ����ʡ����ӷ���ʽ�����ӹ��桢�����ƶϵ����֪ʶ��


 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д� Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��A��B��C��D������Һ���������ռ���ᡢ̼����李��Ȼ�ͭ��Һ�е�ijһ�֡���ȡ����������������ϣ�����A��B�������ɫ��״�������ɣ� C ������B��D��Ͼ�����ɫ�����������������ش��������⣺
��1��B��C�ֱ��� ��
��2����A�еμ�B�����ӷ���ʽ��
��C�еμ�D�����ӷ���ʽ��
��3������B��C��ϣ�����________�����ӷ���ʽ��_______________________________��
��4������������Ӧ������________________��Ӧ(�������Ӧ����)����Ӧ�����������IJ�ͬ���� ����ͬ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Na+Ũ��Ϊ0.5mol/L��ij������Һ�У������ܺ����±��е����������ӣ�
| ������ | K+��Ag+��Mg2+��Ba2+ |
| ������ | NO3-��CO32-��SiO32-��SO42- |
| ��� | ʵ������ | ʵ���� |
| �� | �����Һ�м�������ϡHCl | ������ɫ�������ų�0.56L���� |
| �� | ����ķ�Ӧ���Һ���ˣ��Գ���ϴ�ӡ����������أ��������ù������� | ��������Ϊ2.4g |
| �� | �ڢ����Һ�еμ�BaC12��Һ | ���������� |
| ������ | NO3- | CO32- | SiO32- | SO42- |
| c/mol��L-1 | | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijǿ������ҺX�����ܺ���Al3+��Ba2+��NH4+��Fe2+��Fe3+��CO32-��SO42����SiO32����NO3���е�һ�ֻ������ӣ�ȡ����Һ����ʵ�飬������ת������ͼ����Ӧ��������һ�������Ǻ���ɫ��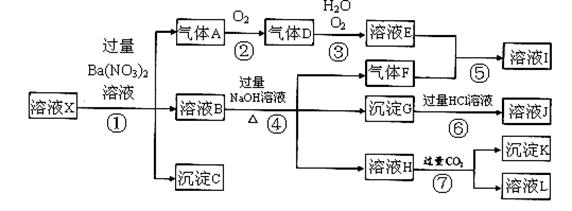
��ش��������⣺
��1����ǿ�������������ж���ҺX��һ�������ڵ������� ��
��2����ҺX�У�����NO3�����ж�һ����ȷ���� ��
a��һ���� b��һ��û�� c��������
��3����������A�����ӷ���ʽΪ ��
��4��ת��������Ӧ������Ϊ ��
��5��ת�����в���H�����ӷ���ʽΪ ��
��6����ת�����У�D��H2O��O2��������ǡ�÷������Ϸ�Ӧ����E����Ӧ��D��O2�����ʵ���֮��
Ϊ ��
��7���Բ���ȷ���Ƿ���ڵ����ӣ�������ȡX��Һ������������Һ�е�һ�֣����������жϣ����Լ������ ��
��NaOH��Һ�� ��KSCN��Һ�� ��ʯ���Լ��� ��pH��ֽ����KMnO4��Һ��
����ˮ��KSCN�Ļ����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ɫ��ҺX����Na+��Ag+��Ba2+��Al3+��AlO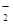 ��MnO
��MnO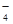 ��CO
��CO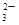 ��SO
��SO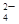 �е�������������ɣ�ȡ��Һ������ͼʵ�飺
�е�������������ɣ�ȡ��Һ������ͼʵ�飺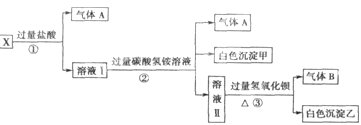
��1����ɫ�������� ��
��2��X��Һ��һ�����ڵ������� ��
��3����ɫ��������һ���У� �������� ��
��4����������������A������������Bͨ��ˮ�У�д����Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ��������·���ʽ��
��1��������������������Ļ�ѧ����ʽ��_________________________________________��
��2���Ȼ�����Һ��ͨ��SO2��������ӷ���ʽ��_______________ ____________________��
��3��ƫ��������Һ��ͨ�����CO2�����ӷ���ʽ��_________________________________��
��4��̼���ʺ�Ũ���ᷴӦ�Ļ�ѧ����ʽ��_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijʵ��С��Ϊ̽��ClO����I2��SO42-�����������µ�������ǿ�������ʵ�����£�
ʵ��٣��ڵ��۵⻯����Һ�м�����������������Һ��������������ϡ���ᣬ��Һ����������
ʵ��ڣ���ʵ��ٵ���Һ�м���4 mL 0.5 mol/L������������Һ����ɫǡ����ȫ��ȥ��
(1)д��ʵ����з�����Ӧ�����ӷ���ʽ��______________________________________________��
(2)ʵ��ڵĻ�ѧ��Ӧ��ת�Ƶ��ӵ����ʵ�����________��
(3)����ʵ��˵����������������ClO����I2��SO42-��������������ǿ��˳����________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(10��)����������ӷ���ʽ
��1�������ữ�ĸ��������Һ����ᷴӦ
��2����������������ᷴӦ
��3��������Һ����۵⻯����Һ����ڿ����з���һ��ʱ��
��4��K2Cr2O7����Һ�д��ڵ�ƽ��
��5���Ȼ��������軯����Һ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������з�Ӧ��ʵ����X��Y2+��X2+��Y����Z��2H2O(��)��Z(OH)2��H2����
��Z2+�����Ա�X2+��������Y��W�缫��ɵĵ�أ��缫��ӦΪW2+��2e-��W��
Y��2e-��Y2+����֪X��Y��Z��W�Ļ�ԭ����ǿ������˳��Ϊ
| A��X>Z>Y>W | B��Z>W>X>Y | C��Z>Y>X>W | D��Z>X>Y>W |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com