
����Ŀ��ij��ͬѧ������ʵ��̽��Fe2+��Fe3+�����ʡ��ش��������⣺
��1���քeȡһ�����Ȼ������Ȼ����������������Ƴ�0.1mol/L����Һ����FeCl2��Һ�������������м����Ŀ����___________��
��2������ͬѧȡ2mLFeCl2��Һ�����뼸����ˮ���ټ���1��KSCN��Һ����Һ��죬˵��Cl2�ɽ�Fe2+������FeCl2��Һ����ˮ��Ӧ�����ӷ���ʽΪ____________��
��3������ͬѧ��Ϊ�����ʵ�鲻������������ͬѧ��2mLFeCl2��Һ���ȼ���0.5mLú��������Һ�������μ��뼸����ˮ��1��KSCN��Һ����Һ��죬ú�͵�������__________��
��4������ͬѧȡ10mL0.1mol/LKI��Һ������6mL0.1mol/LFeCl3��Һ��ϡ��քeȡ2mL����Һ��3֧����н�������ʵ�飺
����һ֧�Թ��м���1mLCCl4�����������CCl4������ɫ��
���ڶ�֧�Թ��м���1��K3[Fe(CN)6]��Һ��������ɫ������
������֧�Թ��м���1��KSCN��Һ����Һ��졣
ʵ��ڼ����������_______(�����ӷ���)��ʵ��ٺ�ʵ���˵������I-���������������Һ���Ժ���_____(�����ӷ���)���ɴ˿���֤����������ԭ��ӦΪ___________��
��5������ͬѧ��ʢ��H2O2��Һ���Թ��м��뼸���ữ��FeCl2��Һ����Һ����ػ�ɫ��������Ӧ�����ӷ���ʽΪ________��һ��ʱ�������Һ�������ݳ��֣������ȣ�����к��ɫ�������ɣ��������ݵ�ԭ����__________�����ɳ�����ԭ����______________(��ƽ���ƶ�ԭ������)��
��6��ij����������(FexO)1.52g������������������������Һͨ���״����112mLCl2��ǡ�ý�Fe2+��ȫ��������xֵΪ________��
���𰸡� ��ֹFe2+������ 2Fe2++Cl2=2Fe3++2Cl- ��������(�ų�������ʵ���Ӱ��) Fe2+ Fe3+ ���淴Ӧ 2Fe2++H2O2+2H+=2Fe3++2H2O Fe3+��H2O2�ֽ����O2 H2O2�ֽⷴӦ���ȣ��ٽ�Fe3+��ˮ��ƽ�������ƶ� 0.80
�����������������⿼��Fe2+��Fe3+������̽�������ӷ���ʽ����д�����淴Ӧ��̽�����������������ˮ��ƽ���Ӱ��ͻ�ѧ���㡣
��1��FeCl2�ױ�������O2������������Fe����Fe3+��Ӧ����Fe2+��
��2��FeCl2��Һ����ˮ��Ӧ�����ӷ���ʽΪCl2+2Fe2+=2Fe3++2Cl-��
��3��ú��������ˮ��ú�͵��ܶȱ�ˮС��ú�͵������Ǹ���������
��4��ʵ����������K3[Fe��CN��6]������ɫ������������Fe2+��ʵ����������KSCN��Һ����Һ�������˵����Ӧ�����Һ���Ժ���Fe3+������I-����ʱ��Һ���Ժ�Fe3+���ɴ�˵����������ԭ��ӦΪ���淴Ӧ��
��5��H2O2���ữ��FeCl2��Һ��Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��һ��ʱ�����Һ�������ݳ��֣�H2O2��Fe3+���·ֽ����O2�����ɵĺ��ɫFe��OH��3������Fe3+ˮ��ƽ����ƶ����͡�
��6�����ݵ�ʧ�����غ���ʽ��
�������1������FeCl2�ױ�������O2����������������Fe����Fe3+��Ӧ����Fe2+����FeCl2��Һ�м���������м�ɷ�ֹFe2+����������FeCl2��Һ�м���������м��Ŀ���ǣ���ֹFe2+��������
��2����ˮ��FeCl2������FeCl3��FeCl2��Һ����ˮ��Ӧ�����ӷ���ʽΪCl2+2Fe2+=2Fe3++2Cl-��
��3��ú��������ˮ��ú�͵��ܶȱ�ˮС��ú�ͽ�FeCl2��Һ������������ų�O2��ʵ���Ӱ�졣ú�͵������ǣ������������ų�O2��ʵ���Ӱ�죩��
��4������ʵ���١�CCl4������ɫ����˵����Ӧ����I2������ʵ����������K3[Fe��CN��6]������ɫ��������˵����Ӧ����Fe2+������ʵ����������KSCN��Һ����Һ�������˵����Ӧ�����Һ���Ժ���Fe3+��ʵ����������K3[Fe��CN��6]������ɫ������������Fe2+��ʵ������ʵ����˵������I-����������£���Һ���Ժ�Fe3+���ɴ�˵����������ԭ��ӦΪ���淴Ӧ��
��5����ʢ��H2O2��Һ���Թ��м��뼸���ữ��FeCl2��Һ����Һ��Ϊ�ػ�ɫ��˵��Fe2+��H2O2������Fe3+��������Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��һ��ʱ�����Һ�������ݳ��֣����ɵ�Fe3+��H2O2�ֽ����O2���������ݵ�ԭ���ǣ�Fe3+��H2O2�ֽ����O2�����ɵĺ��ɫ����ΪFe��OH��3�����ɳ�����ԭ���ǣ�����Һ��Fe3+����ˮ��ƽ����Fe3++3H2O![]() Fe��OH��3+3H+��������ˮ��Ϊ���ȷ�Ӧ��H2O2�ֽ���ȣ��¶����ߣ��ٽ�Fe3+��ˮ��ƽ�������ƶ�����Fe��OH��3������
Fe��OH��3+3H+��������ˮ��Ϊ���ȷ�Ӧ��H2O2�ֽ���ȣ��¶����ߣ��ٽ�Fe3+��ˮ��ƽ�������ƶ�����Fe��OH��3������
��6��n��Cl2��=![]() =0.005mol��FexO��Fe��ƽ����̬Ϊ+
=0.005mol��FexO��Fe��ƽ����̬Ϊ+![]() ��ͨ��Cl2��FeԪ��ȫ��ת��ΪFe3+�����ݵ�ʧ�����غ㣬
��ͨ��Cl2��FeԪ��ȫ��ת��ΪFe3+�����ݵ�ʧ�����غ㣬![]() x
x![]() ��3-
��3-![]() ��=0.005mol
��=0.005mol![]() 2�����x=0.80��
2�����x=0.80��


 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£�A��B��C���������о�����ͬһ����Ӧ��N2(g)+3H2(g) ![]() 2NH3(g) ��H =�� Q kJ��mol��1������ͬ��ʱ����ڣ�����������ݣ�
2NH3(g) ��H =�� Q kJ��mol��1������ͬ��ʱ����ڣ�����������ݣ�
���� | A | B | C |
��Ӧ����/mol��L-1��min-1 | v(H2) = 3 | v(N2) = 3 | v(NH3) = 4 |
�����������ų�������Q�Ĵ�С��ϵΪ�� ��
A. B > C > A B. A > B > C C. C > A > B D. B > A > C
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. ����ʽΪC9H10O2��ͬ���칹����������������һ���������������6��
B. ���������ȶ������ܷ���������Ӧ
C. ������ϩ����ʹ��ˮ��ɫ������ɫ������ͬ
D. ���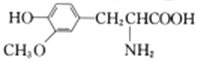 ��
��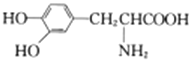 ��Ϊͬϵ��
��Ϊͬϵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���������������
A. ������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫС
B. �ζ�ǰ�ζ����������ݣ��յ����ʱ�����ݣ��������ƫС
C. ����ʪ��pH��ֽ��ϡ����Һ��pH���ⶨֵƫС
D. �ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��������������У������¶�ֵƫС
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з��ӻ�����������ԭ�Ӽ۲���ӶԼ��ι���Ϊ�������ҷ��ӻ����ӿռ�Ĺ���ΪV�ε��ǣ� ��
A. NH4+ B. PH3 C. H3O+ D. OF2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��ij��Һ�еμ�Ba(OH)2��Һʱ�����������ʵ�����Ba(OH)2�����ʵ����ı仯��ϵ������Һ�ijɷֿ�����
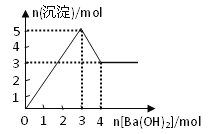
A. MgSO4 B. KAl(SO4)2 C. Al2(SO4)3 D. NaAlO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
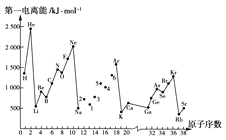
����Ŀ����һ������I1��ָ��̬ԭ��X(g)ʧȥһ�����ӳ�Ϊ��̬������X��(g)����������������ͼ�Dz���Ԫ��ԭ�ӵĵ�һ������I1��ԭ�������仯������ͼ��
��ش��������⣺
(1)���������ͼ��ͬ����Ԫ�ص�һ�����ܵı仯���ɣ���Na��Ar֮���Ԫ���ö�����������������������ͼ��________��
(2)����ͼ������֪��ͬһ����Ԫ��ԭ�ӵĵ�һ������I1�ı仯������_________��
(3)��ͼ��5��Ԫ�������ڱ��е�λ����_________________________________��
(4)��ͼ��4��5��6������Ԫ�ص���̬�⻯��Ļ�ѧʽ�ֱ�Ϊ__________________��
(5)��ͼ��1��6��Ԫ���У�����������Ӧˮ������������ǿ����_______�����Ի�������______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ����д��ȷ����
A. ������ˮ��Ӧ��Cl2 + H2O�� 2H��+ Cl��+ ClO-
B. ʳ���백ˮ��Ӧ��NH3��H2O��H��= NH4����H2O
C. NaHCO3��Һ�м�����Ca(OH)2��Һ:2HCO3-+Ca2++2OH-=CaCO3��+2H2O+CO32-
D. ��Ba(OH)2��Һ�μ�NaHSO4��Һ��Ba2��ǡ�ó�����Ba2����2H����2OH����SO42�� = BaSO4����2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ����Ҫ���ռ��������0.5 mol��L-1��NaOH��Һ490mL��ʵ�����ṩ�������������ձ� ��100 mL��Ͳ ��ҩ�� �ܲ����� ��������ƽ�������룩����ش��������⣺
��1������������Ҫ��ȡNaOH���������Ϊ____________��
��2������ʱ������ʹ�õ�������____________������ţ�����ȱ�ٵ������� _______________��______________�������������ƣ�
��3������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ������ֻ��һ�Σ�__________��
A��������ˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B����ʢ��NaOH������ձ��м�������ˮ�ܽ�
C�����ձ�������ȴ����Һ�ز�����ע������ƿ��
D��������ƿ�ǽ����������µߵ���ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��4��ʵ�������õ��������������÷ֱ��ǣ�������________��������________��
��5��������������������н�����������ҺŨ��ƫ�ߵ���_______________��
������ƿʵ��ǰ������ˮϴ�ɾ�����δ���
�ڶ��ݹ۲�Һ��ʱ����
�����ƹ�������©�ˣ�3���в���A
�ܼ�����ˮʱ���������˿̶�
��6����ʵ������г��֣�5���Т���������㽫��δ�����______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com