

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ����� | Ԥ����������� |
| ����1���õι�ȡһ����3mol��L-1H2SO4���Թ��У�������������ӳ�ȥ�������е����� |  |
| ����2����ҩ��ȡ������Ʒ���Թ��У��õιܼ���������ȥ������3mol��L-1H2SO4�����ȣ���ַ�Ӧ��A��Һ | �����ܽ⣬��Һ��ɫ�б仯 |
| ����3��ȡ����A��Һ���Թ��У��μ�1��2��20%KSCN��Һ���� | �� |
| ����4����ȡ����A��Һ���Թ��У��μ�1��2��0.2mol��L-1KMnO4��Һ���� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
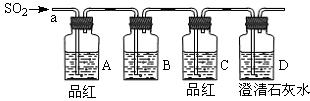
| A��NaCl��Һ | B������KMnO4��Һ | C������ | D������ʯ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
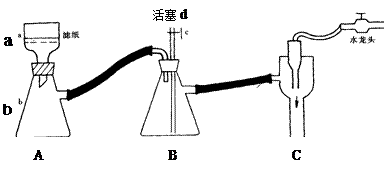
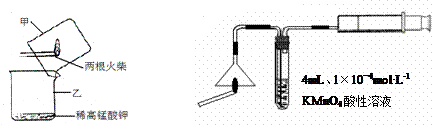

|
|
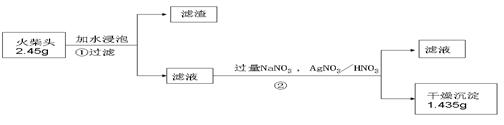
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 �ڻ���Ƥ�ܾ�����ȥ����ɸ�ʵ�飬����������Ϊ�����ң�����ȷ������˳���ǣ�
�ڻ���Ƥ�ܾ�����ȥ����ɸ�ʵ�飬����������Ϊ�����ң�����ȷ������˳���ǣ�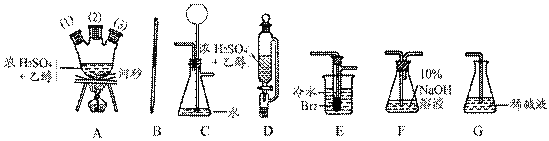
 ____________________��
____________________�� �飬��Ӧ��������ʵ��ǰ����������ɴ˵õ���ϩ������
�飬��Ӧ��������ʵ��ǰ����������ɴ˵õ���ϩ������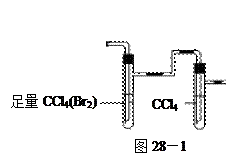 ����II����E��������װ�ò��������ͼ28��2��ʾװ�ý���ʵ�飬��Ӧ���������ϩ��������ɴ������ϩ������
����II����E��������װ�ò��������ͼ28��2��ʾװ�ý���ʵ�飬��Ӧ���������ϩ��������ɴ������ϩ������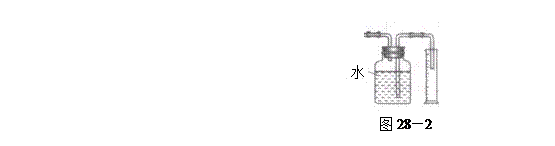
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
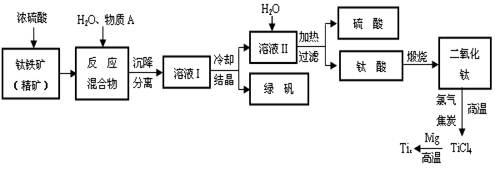
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com