
(15 ��) �������ƣ�NaClO2����һ����Ҫ������������Ҫ����ˮ��ɰ�ǡ���֬��Ư����ɱ������������ȡ�������ƵĹ������̣�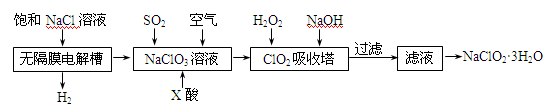
��֪����NaClO2���ܽ�������¶����߶������ʵ������¿ɽᾧ������
��ClO2����ֻ�ܱ�����ϡ��״̬���Է�ֹ��ը�Էֽ⣬�����ֺϳ����á�
��ClO2���������Ժͼ�����Һ�в����ȶ����ڡ�
��1������Ĥ�����г������һ��ʱ�������������NaClO3����д�������ĵ缫��Ӧ����ʽ�� ��
��2����Ӧ����ClO2������ҪX���ữ��ԭ��Ϊ�� ��X��Ϊ ��
��3���������ڵ��¶Ȳ��ܹ��ߵ�ԭ��Ϊ��
��4����������ClO2���Ż�ԭ���IJ�ͬ����Һ����Եı仯�ɱ���ԭΪClO2����Cl����ClO2��S2����ԭΪClO2����Cl����ת��������ҺpH�Ĺ�ϵ����ͼ��ʾ��
��д��pH��2ʱClO2��S2����Ӧ�����ӷ���ʽ�� ��
��5���ڶ�����NaClO3��Һ��ͨSO2��ͬʱͨ�������ԭ��Ϊ�� ��
��6������Һ�еõ�NaClO2��3H2O�־���IJ�������Ϊ
�� �� ��
(15 ��)
(1) Cl��+6e��+6OH��==ClO3��+3H2O��2�֣�
(2) ClO2ֻ�������Ի����д��ڡ���2�֣� ���ᡣ��2�֣�
(3)��ֹH2O2�ֽ⣨2�֣�
(4) 2ClO2+5S2��+ 8H+ ==2Cl��+5S��+4H2O ��2�֣�
(5)ϡ��ClO2���壬��ֹ��ը����2�֣�
(6)����Ũ������ȴ�ᾧ�����ˡ���3�֣�
���������������1�����������ʵõ����ӣ��ʵ缫����ʽΪCl��+6e��+6OH��==ClO3��+3H2O��
��2��ClO2���������Ժͼ�����Һ�в����ȶ����ڣ���ClO2����ֻ�������Ի����д��ڣ���Ϊ���ᡣ
��3���¶Ȳ��ܹ��ߵ�ԭ��Ϊ��ֹH2O2�ֽ⡣
��4������ͼ����ʾ��pH��2ʱ��ClO2ת��ΪCl���������ӷ���ʽΪ2ClO2+5S2��+ 8H+ ==2Cl��+5S��+4H2O��
��5��ClO2����ֻ�ܱ�����ϡ��״̬���Է�ֹ��ը�Էֽ⣬��ͨSO2��ͬʱͨ�������ԭ��Ϊϡ��ClO2���壬��ֹ��ը��
��6�����������һ�㲽��Ϊ����Ũ������ȴ�ᾧ�����ˡ�ע�������Ŀ��ơ�
���㣺��ѧʵ�� ���ԭ�� ������������
�������������й�ʵ�鷽������ƺ����۵Ŀ��飬Ҫ��ѧ����Ϥ��ʵ������ݼ�ԭ�����ܹ�����ͬѧ�ǽ��з������⡢��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(��С��15��)�ö������ȣ�ClO2���������ƣ�Na2FeO4Ħ������Ϊ166 g��mol��1�������;�ˮ�������ͳ�ľ�ˮ��Cl2�Ե�ˮ���������dz�������ˮ�����¼�����ClO2��Na2FeO4��ˮ���������зֱ𱻻�ԭΪCl����Fe3����
(1)����Ե�λ���������������õ��ĵ���������ʾ����Ч�ʣ���ô��ClO2��Na2FeO4��Cl2��������ɱ����������Ч���ɴ�С��˳���� �� �� ��
(2)������֮�����ܾ�ˮ��������������ǿ�������⣬��һ��ԭ������ǣ�
��

(3)����������һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11.0�棬������ˮ��ClO2���Կ����������ᣨHClO2�������ᣨHClO3���Ļ����������ҵ�����Գ�ʪ��KClO3�Ͳ�����60��ʱ��Ӧ�Ƶá�ijѧ������ͼ��ʾ��װ��ģ�ҵ��ȡ���ռ�ClO2������AΪClO2�ķ���װ�ã�BΪClO2������װ�ã�CΪβ������װ�á����ʣ�
��A���ֻ�Ӧ�����¶ȿ��ƣ���ˮԡ���ȣ�װ�ã�B���ֻ�Ӧ����ʲôװ�� ��
��C��Ӧװ���Լ�Ϊ ��C�з�����Ӧ�Ļ�ѧ����ʽΪ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�γ��и�����ѧ��ѧ����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(15 ��) �������ƣ�NaClO2����һ����Ҫ������������Ҫ����ˮ��ɰ�ǡ���֬��Ư����ɱ������������ȡ�������ƵĹ������̣�
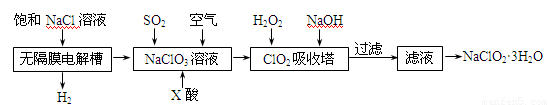
��֪����NaClO2���ܽ�������¶����߶������ʵ������¿ɽᾧ������
��ClO2����ֻ�ܱ�����ϡ��״̬���Է�ֹ��ը�Էֽ⣬�����ֺϳ����á�
��ClO2���������Ժͼ�����Һ�в����ȶ����ڡ�
��1������Ĥ�����г������һ��ʱ�������������NaClO3����д�������ĵ缫��Ӧ����ʽ�� ��
��2����Ӧ����ClO2������ҪX���ữ��ԭ��Ϊ�� ��X��Ϊ ��
��3���������ڵ��¶Ȳ��ܹ��ߵ�ԭ��Ϊ��
��4����������ClO2���Ż�ԭ���IJ�ͬ����Һ����Եı仯�ɱ���ԭΪClO2����Cl����ClO2��S2����ԭΪClO2����Cl����ת��������ҺpH�Ĺ�ϵ����ͼ��ʾ��

��д��pH��2ʱClO2��S2����Ӧ�����ӷ���ʽ�� ��
��5���ڶ�����NaClO3��Һ��ͨSO2��ͬʱͨ�������ԭ��Ϊ�� ��
��6������Һ�еõ�NaClO2��3H2O�־���IJ�������Ϊ
�� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(��С��15��)�ö������ȣ�ClO2���������ƣ�Na2FeO4Ħ������Ϊ166 g��mol��1�������;�ˮ�������ͳ�ľ�ˮ��Cl2�Ե�ˮ���������dz�������ˮ�����¼�����ClO2��Na2FeO4��ˮ���������зֱ𱻻�ԭΪCl����Fe3����
(1)����Ե�λ���������������õ��ĵ���������ʾ����Ч�ʣ���ô��ClO2��Na2FeO4��Cl2��������ɱ����������Ч���ɴ�С��˳���� �� �� ��
(2)������֮�����ܾ�ˮ��������������ǿ�������⣬��һ��ԭ������ǣ� ��
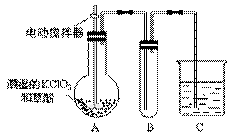
(3)����������һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11.0�棬������ˮ��ClO2���Կ����������ᣨHClO2�������ᣨHClO3���Ļ����������ҵ�����Գ�ʪ��KClO3�Ͳ�����60��ʱ��Ӧ�Ƶá�ijѧ������ͼ��ʾ��װ��ģ�ҵ��ȡ���ռ�ClO2������AΪClO2�ķ���װ�ã�BΪClO2������װ�ã�CΪβ������װ�á����ʣ�
��A���ֻ�Ӧ�����¶ȿ��ƣ���ˮԡ���ȣ�װ�ã�B���ֻ�Ӧ����ʲôװ�� ��
��C��Ӧװ���Լ�Ϊ ��C�з�����Ӧ�Ļ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com