
ŌŚĪĀ¶Č”¢ČŻ»żĻąĶ¬µÄ3øöĆܱÕČŻĘ÷ÖŠ£¬°“²»Ķ¬·½Ź½Ķ¶Čė·“Ó¦Īļ£¬±£³ÖŗćĪĀ”¢ŗćČŻ£¬²āµĆ·“Ó¦“ļµ½Ę½ŗāŹ±µÄÓŠ¹ŲŹż¾ŻČēĻĀ[ŅŃÖŖN2(g)£«3H2(g)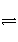
 2NH3(g)””¦¤H£½£92.4 kJ”¤mol£1]£ŗ
2NH3(g)””¦¤H£½£92.4 kJ”¤mol£1]£ŗ
| ČŻĘ÷ | ¼× | ŅŅ | ±ū |
| ·“Ó¦ĪļĶ¶ČėĮæ | 1 mol N2”¢ 3 mol H2 | 2 mol NH3 | 4 mol NH3 |
| NH3µÄÅØ¶Č (mol”¤L£1) | c1 | c2 | c3 |
| ·“Ó¦µÄÄÜĮæ±ä»Æ | ·Å³öa kJ | ĪüŹÕb kJ | ĪüŹÕc kJ |
| ĢåĻµŃ¹Ēæ(Pa) | p1 | p2 | p3 |
| ·“Ó¦Īļ×Ŗ»ÆĀŹ | ¦Į1 | ¦Į2 | ¦Į3 |
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
A£®2c1>c3””””””””””””””””B£®a£«b£½92.4
C£®2p2<p3 D£®¦Į1£«¦Į3>1
B””[²ÉÓĆ”°Ņ»±ßµ¹”±µÄ·½·ØÖŖ¼×”¢ŅŅŹĒĮ½øöĻąĶ¬µÄ×°ÖĆ£¬ĒŅ¼×ÖŠÉś³ÉµÄNH3ÓėŅŅÖŠ·Ö½āµÄNH3µÄŗĶĪŖ2 mol£¬ŌņBĻīÕżČ·£»“ĖŹ±c1£½c2£¬¦Į1£«¦Į2£½1”£¶ų±ūÖŠ¼ÓČėµÄNH3ŹĒŅŅÖŠµÄĮ½±¶£¬æŖŹ¼Ź±c3£½2c2£¬µ«±ūÖŠŃ¹ĒæŅąŹĒŅŅÖŠµÄĮ½±¶£¬Ōö“óŃ¹Ēæ£¬Ę½ŗāÕżĻņŅĘ¶Æ£¬ŌņĘ½ŗāŹ±2c1<c3,2p2>p3£¬A”¢C“ķĪó£»Ōö“óŃ¹Ē棬²»ĄūÓŚNH3µÄ·Ö½ā£¬Ōņ¦Į2>¦Į3£¬¹Ź¦Į1£«¦Į3<1£¬D“ķĪó”£]


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹµŃéŹŅÖʱøĀČĘųµÄ×°ÖĆČēĻĀĶ¼”£Ķ¼ÖŠÉę¼°ĘųĢå·¢Éś”¢³żŌÓ”¢øÉŌļ”¢ŹÕ¼Æ”¢
Ī²Ęų“¦Ąķ×°ÖĆ£¬ĘäÖŠ“ķĪóµÄŹĒ (””””)
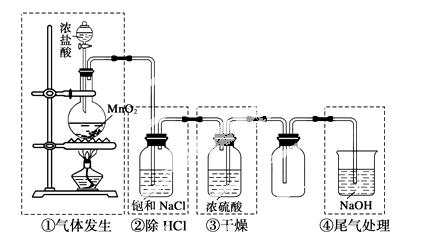
A£®¢Ł B£®¢Ś C£®¢Ū D£®¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹö“ķĪóµÄŹĒ (””””)
A£®ÉśĢśÖŠŗ¬ÓŠĢ¼£¬æ¹øÆŹ“ÄÜĮ¦±Č“æĢśČõ
B£®ÓĆĪżŗø½ÓµÄĢśÖŹĘ÷¼ž£¬ŗø½Ó“¦Ņ×ÉśŠā
C£®ŌŚĢśÖĘĘ·ÉĻ¶ĘĶŹ±£¬¶Ę¼žĪŖŃō¼«£¬ĶŃĪĪŖµē¶ĘŅŗ
D£®Ģś¹ÜÉĻĻāĒ¶Šææ飬Ģś¹Ü²»Ņ×±»øÆŹ“
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚŗćĪĀ”¢ŗćČŻµÄĢõ¼žĻĀ£¬ÓŠ·“Ó¦2A(g)£«2B(g)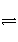
 C(g)£«3D(g)£¬ĻÖ“ÓĮ½ĢõĶ¾¾¶·Ö±š½ØĮ¢Ę½ŗā£¬Ķ¾¾¶¢ń£ŗA”¢BµÄĘšŹ¼ÅØ¶Č¾łĪŖ2 mol”¤L£1£»Ķ¾¾¶¢ņ£ŗC”¢DµÄĘšŹ¼ÅØ¶Č·Ö±šĪŖ2 mol”¤L£1ŗĶ6 mol”¤L£1£¬ŅŌĻĀŠšŹöÕżČ·µÄŹĒ(””””)
C(g)£«3D(g)£¬ĻÖ“ÓĮ½ĢõĶ¾¾¶·Ö±š½ØĮ¢Ę½ŗā£¬Ķ¾¾¶¢ń£ŗA”¢BµÄĘšŹ¼ÅØ¶Č¾łĪŖ2 mol”¤L£1£»Ķ¾¾¶¢ņ£ŗC”¢DµÄĘšŹ¼ÅØ¶Č·Ö±šĪŖ2 mol”¤L£1ŗĶ6 mol”¤L£1£¬ŅŌĻĀŠšŹöÕżČ·µÄŹĒ(””””)
A£®Į½Ķ¾¾¶×īÖÕ“ļµ½Ę½ŗāŹ±£¬ĢåĻµÄŚ»ģŗĻĘųĢåµÄ°Ł·Ö×é³ÉĻąĶ¬
B£®Į½Ķ¾¾¶×īÖÕ“ļµ½Ę½ŗāŹ±£¬ĢåĻµÄŚ»ģŗĻĘųĢåµÄ°Ł·Ö×é³É²»Ķ¬
C£®“ļµ½Ę½ŗāŹ±£¬Ķ¾¾¶¢ńµÄ·“Ó¦ĖŁĀŹv(A)µČÓŚĶ¾¾¶¢ņµÄ·“Ó¦ĖŁĀŹv(A)
D£®“ļµ½Ę½ŗāŹ±£¬Ķ¾¾¶¢ńĖłµĆ»ģŗĻĘųĢåµÄĆܶȵČÓŚĶ¾¾¶¢ņĖłµĆ»ģŗĻĘųĢåµÄĆܶČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚŅ»ÕęæÕĆܱÕČŻĘ÷ÖŠŹ¢ÓŠ1 mol PCl5£¬¼ÓČȵ½200”ę£¬·¢Éś·“Ó¦£ŗPCl5(g)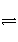
 PCl3(g)£«Cl2(g)£¬·“Ó¦“ļµ½Ę½ŗāŹ±£¬PCl5ŌŚ»ģŗĻĘųĢåÖŠµÄĢå»ż·ÖŹżĪŖm%£¬ČōŌŚĻąĶ¬µÄĪĀ¶ČŗĶĻąĶ¬µÄČŻĘ÷ÖŠ£¬ĘšŹ¼Ź±¼ÓČė2 mol PCl5£¬·“Ó¦“ļµ½Ę½ŗāŹ±£¬PCl5ŌŚ»ģŗĻĘųĢåÖŠµÄĢå»ż·ÖŹżĪŖn%£¬ŌņmŗĶnµÄ¹ŲĻµÕżČ·µÄŹĒ(””””)
PCl3(g)£«Cl2(g)£¬·“Ó¦“ļµ½Ę½ŗāŹ±£¬PCl5ŌŚ»ģŗĻĘųĢåÖŠµÄĢå»ż·ÖŹżĪŖm%£¬ČōŌŚĻąĶ¬µÄĪĀ¶ČŗĶĻąĶ¬µÄČŻĘ÷ÖŠ£¬ĘšŹ¼Ź±¼ÓČė2 mol PCl5£¬·“Ó¦“ļµ½Ę½ŗāŹ±£¬PCl5ŌŚ»ģŗĻĘųĢåÖŠµÄĢå»ż·ÖŹżĪŖn%£¬ŌņmŗĶnµÄ¹ŲĻµÕżČ·µÄŹĒ(””””)
A£®m>n B£®m<n C£®m£½n D£®ĪŽ·Ø±Č½Ļ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĄė×Ó·“Ó¦·½³ĢŹ½“ķĪóµÄŹĒ
A£®ĻņNa2SiO3ČÜŅŗÖŠÖšµĪ¼ÓČėÉŁĮæĻ”ŃĪĖį£ŗSiO32£+ 2H+£½H2SiO3£Ø½ŗĢ壩
B£®ĻņNa2S2O3ČÜŅŗÖŠ¼ÓČėĻ”ĮņĖį£ŗ2S2O32££«2H£«£½SO42££«3S”ż£«H2O
C£®½«Cuʬ¼ÓČėĻ”ĻõĖįÖŠ£ŗ3Cu + 8H£«+2NO3££½3Cu2+ +2NO”ü+ 4H2O
D£®ĻņNH4Al(SO4)2ČÜŅŗÖŠ¼ÓČė¹żĮæµÄBa(OH)2Ļ”ČÜŅŗ£ŗ
NH4+ + Al3+ + 2SO42£ + 2Ba2+ + 5OH££½2BaSO4”ż+ NH3”¤H2O + AlO2£+ 2H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¼×“¼ÓÖ³Ę”°Ä¾“¼”±»ņ”°Ä¾¾«”±£¬·Šµć64.7”ę£¬ŹĒĪŽÉ«ÓŠ¾Ę¾«ĘųĪ¶Ņ×»Ó·¢µÄŅŗĢ唣¼×“¼ÓŠ¶¾£¬ĪóŅū5”«10mLÄÜĖ«ÄæŹ§Ć÷£¬“óĮæŅūÓĆ»įµ¼ÖĀĖĄĶö”£¼×“¼ŹĒÖŲŅŖµÄ»Æѧ¹¤Ņµ»ł“”ŌĮĻŗĶŅŗĢåČ¼ĮĻ£¬æÉÓĆÓŚÖĘŌģ¼×Č©ŗĶÅ©Ņ©£¬²¢³£ÓĆ×÷ÓŠ»śĪļµÄŻĶČ”¼ĮŗĶ¾Ę¾«µÄ±äŠŌ¼ĮµČ”£
¢Å¹¤ŅµÉĻæÉĄūÓĆCO2ŗĶH2Éś²ś¼×“¼£¬·½³ĢŹ½ČēĻĀ£ŗ
CO2(g)£«3H2(g) CH3OH(l)£«H2O (g) ”÷H£½Q1kJ”¤mol£1
CH3OH(l)£«H2O (g) ”÷H£½Q1kJ”¤mol£1
ÓÖ²é׏ĮĻµĆÖŖ£ŗ¢ŁCH3OH(l)£«1/2 O2(g) CO2(g)£«2H2(g) ”÷H£½Q2kJ”¤mol£1
CO2(g)£«2H2(g) ”÷H£½Q2kJ”¤mol£1
¢ŚH2O(g)=H2O(l) ”÷H= Q3kJ”¤mol£1£¬Ōņ±ķŹ¾¼×“¼µÄČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
¢Ę¹¤ŅµÉĻæÉÓĆCOŗĶH2O (g) Ą“ŗĻ³ÉCO2 ŗĶH2£¬ŌŁĄūÓĆ¢ÅÖŠ·“Ó¦ŌĄķŗĻ³É¼×“¼”£Ä³ĪĀ¶ČĻĀ£¬½«1molCOŗĶ1.5molH2O³äČė10 L¹Ģ¶ØĆܱÕČŻĘ÷ÖŠ½ųŠŠ»Æѧ·“Ó¦£ŗ
L¹Ģ¶ØĆܱÕČŻĘ÷ÖŠ½ųŠŠ»Æѧ·“Ó¦£ŗ
CO(g)£«H2O(g) CO2(g)£«H2(g) ”÷H£¾0,µ±·“Ó¦½ųŠŠµ½10minŹ±“ļµ½Ę½ŗā£¬“ĖŹ±²āµĆH2ĪŖ0.6 mol”£»Ų“šĻĀĮŠĪŹĢā:
CO2(g)£«H2(g) ”÷H£¾0,µ±·“Ó¦½ųŠŠµ½10minŹ±“ļµ½Ę½ŗā£¬“ĖŹ±²āµĆH2ĪŖ0.6 mol”£»Ų“šĻĀĮŠĪŹĢā:
¢Ł0~10minÄŚH2O(g)µÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ ”£
¢ŚČōĻė¼ÓæģÕż·“Ó¦ĖŁĀŹµÄĶ¬Ź±ĢįøßCOµÄ×Ŗ»ÆĀŹ£¬æÉŅŌ²ÉÓƵķ½·Ø ŹĒ ”£
ŹĒ ”£
a.ÉżøßĪĀ¶Č b.ĖõŠ”ČŻĘ÷µÄĢå»ż
c.Ōö“óH2O (g)µÄÅØ¶Č d.¼ÓČėŹŹµ±µÄ“߻ƼĮ
¢ŪČō±£³ÖĪĀ¶ČČŻ»ż²»±äŌŁĻņĘäÖŠ³äČė1molCOŗĶ0.5molH2O(g)£¬ÖŲŠĀ“ļµ½»ÆŃ§Ę½ŗāדĢ¬Ź±,“ĖŹ±Ę½ŗā»ģŗĻĘųĢåÖŠH2µÄĢå»ż·ÖŹżĪŖ ”£
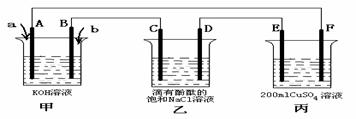
¢Ē¼×“¼Č¼ĮĻµē³ŲŹĒ·ūŗĻĀĢÉ«»ÆѧĄķÄīµÄŠĀŠĶČ¼ĮĻµē³Ų£¬ĻĀĶ¼ŹĒŅŌ¼×“¼Č¼ĮĻµē³Ų£Ø¼×³Ų£©ĪŖµēŌ“µÄµē½ā×°ÖĆ”£ŅŃÖŖ£ŗA”¢B”¢C”¢D”¢E”¢F¶¼ŹĒ¶čŠŌµē¼«£¬±ūÖŠĪŖ0.1 mol/L CuSO4ČÜŅŗ (¼ŁÉč·“Ó¦Ē°ŗóČÜŅŗĢå»ż²»±ä) £¬µ±Ļņ¼×³ŲĶØČėĘųĢåaŗĶbŹ±£¬D¼«ø½½ü³ŹŗģÉ«”£»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Ł aĪļÖŹŹĒ , Aµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
¢Ś ŅŅ×°ÖĆÖŠµÄ×Ü»Æѧ·“Ó¦·½³ĢŹ½ĪŖ ”£
¢Ū µ±ŅŅ×°ÖĆÖŠCµē¼«ŹÕ¼Æµ½224mL(±źæöĻĀ)ĘųĢåŹ±, ±ūÖŠČÜŅŗµÄpH£½ ”£
 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
°ĀŌĖ½šÅĘ”°½šĻāÓń”±»·ŠĪÓńčµÓÉĄ„ĀŲÓńÖĘ³É£¬Ą„ĀŲÓńµÄ³É·Öæɼņµ„æ“³ÉŹĒCa2Mg5Si8O22(OH)2£¬ŌņĘäÓƶžŃõ
 »Æ¹čŗĶ½šŹōŃõ»ÆĪļµÄŠĪŹ½æɱķŹ¾ĪŖ(””””)
»Æ¹čŗĶ½šŹōŃõ»ÆĪļµÄŠĪŹ½æɱķŹ¾ĪŖ(””””)
A£®CaO·MgO·SiO2·H2O
B£®2CaO·5MgO·8SiO2·H2O
C£®2CaO·MgO·SiO2·2H2O
D£®5CaO·2MgO·8SiO2·H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾»Æѧ—Ń”ŠŽ5£ŗÓŠ»ś»Æѧ»ł“””æAŹĒŅ»Č”“ś·¼Ļć»ÆŗĻĪļ£¬Ļą¶Ō·Ö×ÓÖŹĮæĪŖ136£¬·Ö×ÓÖŠÖ»ŗ¬Ģ¼”¢Ēā”¢Ńõ£¬ĘäÖŠŃõµÄŗ¬ĮæĪŖ23.5%”£ŹµŃé±ķĆ÷£ŗ AµÄ·¼»·²ąĮ“ÉĻÖ»ŗ¬Ņ»øö¹ŁÄÜĶÅ£»AŗĶNaOHČÜŅŗ·“Ó¦ŗóĖį»ÆæÉŅŌµĆµ½E£ØC7H6O2£©ŗĶF”£
£Ø1£©A”¢E”¢FµÄ½į¹¹¼ņŹ½”£
£Ø2£©AŗĶNaOHČÜŅŗ·“Ó¦”¢Ėį»ÆµÄŹµŃé×°ÖĆČēĻĀ£ŗ
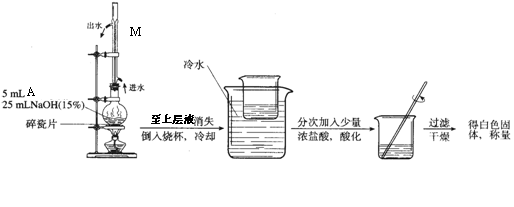

 Š“³öAÓėNaOHČÜŅŗ·“Ó¦µÄ»Æѧ·“Ó¦·½³ĢŹ½ ”£
Š“³öAÓėNaOHČÜŅŗ·“Ó¦µÄ»Æѧ·“Ó¦·½³ĢŹ½ ”£
Š“³öŹµŃé×°ÖĆÖŠMµÄĆū³ĘŗĶ×÷ÓĆ ”£
£Ø3£©AÓŠ¶ąÖÖĶ¬·ÖŅģ¹¹Ģ壬·ūŗĻĻĀĮŠĢõ¼žµÄ½į¹¹¹²ÓŠ ÖÖ£¬
¢ŁæÉŅŌ·¢ÉśŅų¾µ·“Ó¦ ¢ŚŹōÓŚ·¼Ļć×å»ÆŗĻĪļ£¬²»¾ß±øĘäĖü»·×“½į¹¹
¢ŪæÉŅŌÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦ ¢Ü²»ÄÜÓėFeCl3·¢ÉśĻŌÉ«·“Ó¦
ĒėŠ“³öĘäÖŠŗĖ“Ź²ÕńĒāĘ×ÓŠ5øöĪüŹÕ·åµÄAµÄ½į¹¹µÄ½į¹¹¼ņŹ½£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com