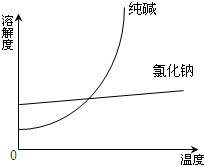
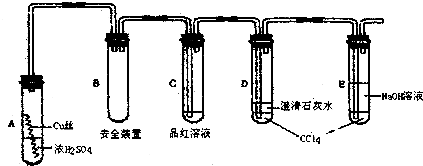
I������ʵ��������Լ����淽����ʵ�����¹ʴ�����һ����ȷ����
AB
AB
������ţ���
A��������Ũ��Һմ��Ƥ���ϣ�Ҫ�����ô���ˮ��ϴ��Ȼ��Ϳ��������Һ
B���Ʊ�������������ʱ��Ӧ���ˮ����εμ�1��2mL���͵�FeC1
3��Һ���������� �ȵ�Һ������ĺ��ɫΪֹ
C���ⶨ��Һ��pHʱ���ýྻ������IJ�����պȡ��Һ����������ˮʪ�����pH��ֽ�ϣ��������ɫ���Ƚ�
D��ʵ�����У�Ũ���ᱣ���ڴ���������ɫϸ���Լ�ƿ��
E����ȥ��������Һ�л��е�NaC1�������ȼ���AgNO
3��Һ��Ȼ�����
F���ڽ��з�Ӧ�Ȳⶨʱ��Ϊ��֤ʵ���ȷ�ԣ����ǿ��Բ�ȡ���¾����ʩ��ʹ������ĭ�����ȱ��µ����á�ʹ��ͭ�ʽ�������н��衢ʹ�õ�������÷�Ӧ��������������ʵ�飬ȡƽ��ֵ
II��ʯ��ʯ����Ҫ�ɷ���̼��ƣ���������Լ40��50%���Ϻõ�ʯ��ʯ��CaOԼ45��53%�������SiO
2��Fe
2O
3��Al
2O
3��MgO�����ʣ��ⶨʯ��ʯ�иƵĺ���ʱ����Ʒ�������ᣬ����������Һ�������Ի���Խ������������ܵIJ���Ƴ��� ��CaC
2O
4?H
2O���������ó������ˡ�ϴ�����������ܽ⣬�ñ����������Һ�ζ����ɵIJ��ᣬͨ���������Ķ�����ϵ���������Ƶĺ������漰�Ļ�ѧ��ӦΪ��H
2C
2O
4+MnO
4-+H
+��Mn
2++CO
2��+H
2O��δ��ƽ����CaC
2O
4 ��������ϸС����մ�ۣ����ڹ��ˣ�Ϊ�˵õ��������ִ�Ľᾧ��ͨ���ں�Ca
2+��������Һ�м��뱥�� ��NH
4��
2C
2O
4������C
2O
42-Ũ�Ⱥܵͣ����������ɳ�������ʱ����Һ�еμӰ�ˮ����Һ��C
2O
42-Ũ�����������Ի�ÿ����Ƚϴִ��CaC
2O
4������������Ϻ�pHӦ��3.5��4.5�������ɱ����������ܸ����������ֲ�ʹCaC
2O
4�ܽ��̫�������ϲ��ϻش��������⣺
��1����Ʒ���������õ��ij�������Ҫ��
SiO2
SiO2
��2������ CaC
2O
4Ҫ������������͵� ��NH
4��
2C
2O
4 ��Һ��Ϊʲô��
���ֽϴ��C2O42-����Ũ�ȣ�ʹCa2+������ȫ
���ֽϴ��C2O42-����Ũ�ȣ�ʹCa2+������ȫ
��3����ҵ��ͨ������0.1%�������Һϴ�ӳ����������������ˮϴ�ӣ���Ŀ���ǣ�
����CaC2O4��ˮ�е��ܽ�ȣ����ٳ�������ʧ������ʵ�����
����CaC2O4��ˮ�е��ܽ�ȣ����ٳ�������ʧ������ʵ�����
��4������ʼ��ȥm g��Ʒ�����ζ���ȥŨ��Ϊc mol/L ��KMnO
4 ��ҺV mL���Ƴ�CaO������ KMnO
4�Ķ��������ϵ����%=
��

 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�