
����ͼװ�õ���������Һ��ȡ������������������������ء��ӿ�ʼͨ��ʱ���ռ�B��C�ݳ������塣1 min ����B�ڵ��������ΪC�ڴ���һ�룬����˵������ȷ����(����)
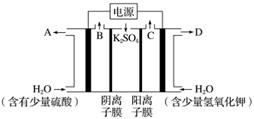
A����Դ���Ϊ����
B���������ĵ缫��Ӧ����ʽΪ2H2O��4e��===O2��4H��
C��D�ڵ�������ҺΪKOH��Һ����Ũ�ȱȸռ�������Ҳ�ʱ��Ũ�ȴ�
D���ڱ�״���£���1 min���C�ڴ��ռ����������B�ڴ��ռ����������2.24 L������0.1NA��SO ͨ��������Ĥ
ͨ��������Ĥ
�𰸡�AD
�������������⣬�缫ӦΪ���Ե缫�����缫Ϊ�������Ҳ�缫Ϊ�������缫��ӦʽΪ
������4OH����4e��===2H2O��O2����2H2O��4e��===O2����4H����
������4H����4e��===2H2����4H2O��4e��===2H2����4OH����
A����B��ȷ��C��Ҳ�����H���ŵ磬����KOH��������Ũ�ȱȸռ�������Ҳ�ʱ��Ũ�ȴ�D������O2Ϊx mol�������H2Ϊ2x mol��
2x��x��0.1 mol��x��0.1 mol
���ĵ�OH��Ϊ0.4 mol������0.2NA��SO ͨ��������Ĥ��
ͨ��������Ĥ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������[CO(NH2)2]�ļ�����Һ��������װ��ʾ��ͼ���£�

�����и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫��
(1)A��Ϊ________���缫��ӦʽΪ_________________________________________��
(2)B��Ϊ________���缫��ӦʽΪ______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���NO�Ʊ�NH4NO3���乤��ԭ����ͼ��ʾ��Ϊʹ������ȫ��ת��ΪNH4NO3���貹������A��A��________��˵�����ɣ�______________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��������(ClO2)Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������Ŀǰ�ѿ������õ�ⷨ��ȡClO2���¹��ա�

��ͼ����ʯī���缫����һ�������µ�ⱥ��ʳ��ˮ��ȡClO2������������ClO2�ĵ缫��ӦʽΪ____________________��
�ڵ��һ��ʱ�䣬�������������������Ϊ112 mL(��״��)ʱ��ֹͣ��⡣ͨ�������ӽ���Ĥ�������ӵ����ʵ���Ϊ________ mol����ƽ���ƶ�ԭ������������pH�����ԭ��________________________________________________________________________
________________________________________________________________________��
(2)Ϊ��״�ȼ�ϵ������ʣ���ѧ�ҷ�����һ��ȼ�ϵ�أ���ص�һ���缫ͨ���������һ���缫ͨ��״����壬������Dz�����Y2O3��ZrO2���壬�ڸ��������ܴ���O2������ع���ʱ������ӦʽΪ______________________________________________
________________________________________________________________________��
���Ըõ��Ϊ��Դ����ʯī���缫���100 mL�����������ӵ���Һ��
| ���� | Cu2�� | H�� | Cl�� | SO |
| c/mol·L��1 | 1 | 4 | 4 | 1 |
���һ��ʱ��������ռ�����ͬ���(��ͬ����)������ʱ(������Һ����ı仯���缫������ܴ��ڵ��ܽ�����)���������ռ������������ʵ���Ϊ________ mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪2SO2(g)��O2(g)??2SO3(g)����H����196.64 kJ·mol��1����һ���¶��£���һ�̶��ݻ����ܱ�������ͨ��2 mol SO2��1 mol O2���ﵽƽ��ʱ�ų�����ΪQ1 kJ����ͬ�������£����������ͨ��2 mol SO3���ﵽƽ��ʱ����������ΪQ2 kJ����Q1��Q2�Ĺ�ϵΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
LiH�����ɴ���ȼ�ϣ���֪���з�Ӧ��
��2Li(s)��H2(g)===2LiH(s)����H����182 kJ·mol��1
��2H2(g)��O2(g)===2H2O(l)����H����572 kJ·mol��1
��4Li(s)��O2(g)===2Li2O(s)����H����1 196 kJ·mol��1
��д��LiH��O2��ȼ�յ��Ȼ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�����ͬ��Ũ�Ⱦ�Ϊ0.2 mol·L��1�������CH3COOH��Һ���ֱ��ˮϡ��10������Һ��pH�ֱ���m��n����m��n�Ĺ�ϵΪ________��
(2)�����ͬ��Ũ�Ⱦ�Ϊ0.2 mol·L��1�������CH3COOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����3����m��n�Ĺ�ϵΪ________________��
(3)�����ͬ��pH������1�������CH3COOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����3����m��n�Ĺ�ϵΪ________________��
(4)�����ͬ��pH������13�İ�ˮ��NaOH��Һ���ֱ��ˮϡ��m����n������Һ��pH�����9����m��n�Ĺ�ϵΪ________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com