
(14��)X��Y��Z��W��G����H���ɶ�����Ԫ����ɣ���������ѧ��ѧ�г��������壬�����������ʣ�
��X��Y��G��ʹʪ�����ɫʯ����ֽ��죬H��ʹʪ��ĺ�ɫʯ����ֽ������Z��W����ʹʪ���ʯ����ֽ��ɫ��
��X��H�����������̣�
��Y���γ��������Ҫ��������ʹƷ����Һ��ɫ��
��Z��W�������ɺ���ɫ���壻
��G��W��ȼ�տ��Բ���Y��H2O��
�ش��������⣺
��1�� H�Ļ�ѧʽ��__________��
ʵ������ȡH�Ļ�ѧ��Ӧ����ʽ��___________________________________________��
��2��Z�Ļ�ѧʽ��________��W�Ļ�ѧʽ��__________________��
��3�����з�����Ӧ�Ļ�ѧ����ʽ��___________________________________________��
��4��ʵ�����Ʊ����ռ������Y���壬�����������¡�װ��A����Y���壬�������������Ӹ������ӿڣ�˳��Ϊa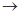
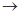
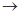
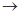
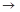 f��
f��
װ��D��������_ ___��װ��E��NaOH��Һ��������__ ____��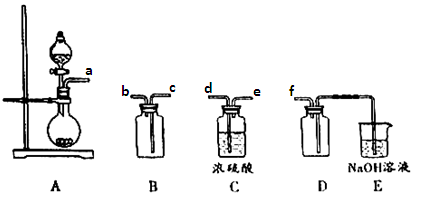
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012�찲��ʡ��ɽ�и�����У������ѧ�Ծ� ���ͣ������
(14��)X��Y��Z��W��Ԫ�����ڱ���ǰ�����ڵij���Ԫ�أ��������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
| Y | ԭ�������������Ǵ��������� |
| Z | ���ʼ��仯�������ɫ��ӦΪ��ɫ |
| W | WԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ������ |
 ��X��һ�ֵ����۵�ܸߣ�Ӳ�Ⱥܴ������ֵ��ʵľ������� ���塣
��X��һ�ֵ����۵�ܸߣ�Ӳ�Ⱥܴ������ֵ��ʵľ������� ���塣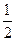 O2(g) ="=" H2O(l)�� ��H= -285.8kJ��mol-1
O2(g) ="=" H2O(l)�� ��H= -285.8kJ��mol-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ��ɽ�и�����У������ѧ�Ծ� ���ͣ������
(14��)X��Y��Z��W��Ԫ�����ڱ���ǰ�����ڵij���Ԫ�أ��������Ϣ���±���
|
Ԫ�� |
�����Ϣ |
|
X |
X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
|
Y |
ԭ�������������Ǵ��������� |
|
Z |
���ʼ��仯�������ɫ��ӦΪ��ɫ |
|
W |
WԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ������ |
�� Xλ��Ԫ�����ڱ��� �塣X��һ�ֵ����۵�ܸߣ�Ӳ�Ⱥܴ������ֵ��ʵľ������� ���塣
�� X��Y�е縺�Խ�ǿ����(��Ԫ�ط��ţ� ��XY2�ĵ���ʽ�� �������д��� ���Ҽ���
��Z2Y2�к��еĻ�ѧ�������� �����������ӵĸ�����Ϊ ��
��W�Ļ�̬ԭ�Ӻ�������Ų�ʽ�� ��
�ɷϾ�ӡˢ��·������W�ĵ���A����H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·���ϵ�A����֪��
A(s)+H2SO4 (aq) == ASO4(aq) + H2(g)�� ��H=+64.4kJ��mol-1
2H2O2(l) == 2H2O(l) + O2(g)�� ��H= -196.4kJ��mol-1
H2(g)+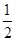 O2(g) == H2O(l)�� ��H= -285.8kJ��mol-1
O2(g) == H2O(l)�� ��H= -285.8kJ��mol-1
��д��A��H2SO4��H2O2��Ӧ����ASO4(aq)��H2O(l)���Ȼ�ѧ����ʽ(A�û�ѧʽ��ʾ)��
�� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�갲��ʡ�Ϸ��и����ڶ��ν�ѧ����������ۻ�ѧ���� ���ͣ������
(14��)X��Y��Z��W��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ���ԭ��������������XԪ�ص�һ�ֺ��ص�������Ϊ12,������Ϊ6;YԪ���Ƕ�ֲ����������ȱ�١����ɵ����ʵ���Ҫ���Ԫ��;Z�Ļ�̬ԭ�Ӻ���9�����������˵���,�Һ�����2��δ�ɶԵ��ӣ���Y��ͬ��;W��һ�ֳ���Ԫ��,�����γ�3��������,����һ���������Ǿ��д��Եĺ�ɫ���塣
(1) ���³�ѹ��Z������_______���壨�����ͣ�������ͨ��______�γɾ���(����������õ����ͣ���Y2�����д���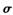 ����
����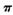 ������֮��Ϊ____________��
������֮��Ϊ____________��
(2) X-H��Y��H���ڼ��Թ��ۼ�,���м��Խ�ǿ�ļ���______(X��Y��Ԫ�ط��ű�ʾ����X�ĵ�һ�����ܱ�Y��______(���С������
(3) д��X������Z������������Ӧ��ˮ�����Ũ��Һ��Ӧ�Ļ�ѧ����ʽ____________________________________________________________
(4) W�Ļ�̬ԭ�ӵ���Χ�����Ų�ʽ��____________��
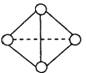
(5) ��֪һ�ַ���Y4���ӽṹ����ͼ��ʾ������1molY��Y����167KJ������,����1molY Y�ų�942KJ��������д����Y4��̬���ӱ��Y2��̬���ӵ��Ȼ�ѧ����ʽ________________________
Y�ų�942KJ��������д����Y4��̬���ӱ��Y2��̬���ӵ��Ȼ�ѧ����ʽ________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011�긣��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(14��)X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ�M�Ƕ������н�������ǿ��Ԫ�ء��ش��������⣺
��1��L��Ԫ�ط���Ϊ ��M��Ԫ�����ڱ��е�λ��Ϊ ��L��MԪ�ص����Ӱ뾶��С˳���� �������ӷ��ű�ʾ����
��2��Z��X��Ԫ�ذ�ԭ����Ŀ��l��3���ɷ���A��A�ĵ���ʽΪ ��
��3������Se��������������Ԫ�أ���Lͬһ���壬Seԭ�ӱ�Lԭ�Ӷ��������Ӳ㣬����������������ˮ���ﻯѧʽΪ ��
��4����X��Z��L����Ԫ����ɵ����ӻ�������ϡNaOH��Һ���ȿ�����ʹ��ɫʯ����ֽ���������壬д���÷�Ӧ�����ӷ���ʽ�� ��
��5��д����M��LԪ����ɵ����ʵĵ���ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com