
ij����С������ͼ����ʵ�飬�Իش��������⡣
��1������ʼʱ����K��a���ӣ���B���ĵ缫��ӦʽΪ ��
��2������ʼʱ����K��b���ӣ���B���ĵ缫��ӦʽΪ ��
�ܷ�Ӧ�����ӷ���ʽΪ ��
��3��������K��b����ʱ������˵����ȷ����(�����) ��
����Һ��Na����B���ƶ�
�ڴ�A�����ݳ���������ʹʪ���KI������ֽ����
�۷�Ӧһ��ʱ����������������ɻָ������ǰ����ʵ�Ũ��
������״����B������2.24L���壬����Һ��ת����0.2mol����
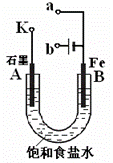
��1��Fe��2e���� Fe2+������������1�֣���
(2)2H+��2e���� H2��(д��2H2O ��2e���� H2��+2OH��Ҳ�����֡�������������1�֡���
2Cl����2H2O  2OH����H2����Cl2����д�ɻ�ѧ����ʽ�����֡�������д�ɡ���⡱��ͨ�硱��������д����������ϲ���1�֣�
2OH����H2����Cl2����д�ɻ�ѧ����ʽ�����֡�������д�ɡ���⡱��ͨ�硱��������д����������ϲ���1�֣�
�ر�˵������������ʽ�С�����д�ɡ������ϲ���1�֡��ڶ���������д�������ϲ���1�֡�
��3���٢� ���ֱ���1��©ѡ��1�֡�2����ѡ���۷֣��������ָ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪Fe��s��+CO2��g�� FeO��s��+CO��g�� ��H=a kJ/mo1��ƽ�ⳣ��ΪK������ڲ�ͬ�¶��£�Kֵ���£�
FeO��s��+CO��g�� ��H=a kJ/mo1��ƽ�ⳣ��ΪK������ڲ�ͬ�¶��£�Kֵ���£�
| �¶�/�� | 500 | 700 | 900 |
| K | 1.00 | 1.47 | 2.40 |
��1����500��ʱ����������Ӧ��CO2��ʼŨ��Ϊ2 mol/L��CO��ƽ��Ũ��Ϊ__________��
��2������ʽ�е�a______________0������ڡ�����С�ڡ����ڡ�����
��3��700��ʱ��������Ӧ�ﵽƽ�⣬Ҫʹ�ø�ƽ�������ƶ���������������ʱ�����Բ�ȡ�Ĵ�ʩ��________������ţ�
a. ��С��Ӧ����� b. ͨ��CO2
c. �����¶ȵ�900�� d. ʹ�ú��ʵĴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.1 mol��þ�������������100 mL 2 mol/L H2SO4��Һ�У�Ȼ ���ٵμ�1 mol/L NaOH��Һ����ش�
���ٵμ�1 mol/L NaOH��Һ����ش�
(1)���ڵμ�NaOH��Һ�Ĺ����У���������m�����NaOH��Һ�����V�仯����ͼ��ʾ����V1��160 mLʱ���������ĩ��n(Mg)��________mol��V2��________ mL��
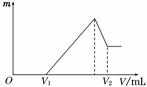
(2)���ڵμ�NaOH��Һ�����У���ʹMg2����Al3���պó�����ȫ�������NaOH��Һ�����V(NaOH)��________ mL��
(3)���������Ϊ0.1 mol������þ�۵����ʵ�������Ϊa����100 mL 2 mol/L�������ܽ�˻������ټ���450 mL 1 mol/L��NaOH��Һ�����ó�������Al(OH)3�������������a��ȡֵ��Χ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ����ʵ���ó��Ľ����У�������ǣ� ��
A����AgCl�����е���ϡKI��Һ��ɫ������ƣ�˵��AgI��AgCl������
B���������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ���壬��������һ����CO32-
C���ȼ��������������ټ���BaCl2��Һ������ɫ��������������һ����SO42-
D������Һ�м���NaOH�ȣ�����ʹʪ��ĺ�ɫʯ����ֽ���������壬��һ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��100mLH2SO4��CuSO4�Ļ��Һ�У���ʯī���缫��⣬�����Ͼ��ռ���2.24L���壨��״���£�����ԭ���Һ�У�Cu2+�����ʵ���Ũ��Ϊ�� ����
A.1mol��L��1 B.2mol��L��1 C.3mol��L��1 D.4mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ǿ������Ҹ�״̬���ܵ������ ( )
(1)�Ȼ�����Һ�� (2)�Ȼ�粒��壻 (3)ͭ�� (4)ʯī��
(5)����NaOH�� (6) ϡ��� (7)���
A��(1)(2)(5)(6) B��(5)
C��(2)(5) D��(1)(3)(4)(5)(6)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��298K��100kPaʱ����֪��2H2O(g)��O2(g)��2H2(g)������H1
Cl2(g)��H2(g)��2HCl(g) ��H2
2Cl2(g)��2H2O(g)��4HCl(g)��O2(g) ��H3
��H3�릤H1�ͦ�H2��Ĺ�ϵ��ȷ���� �� ����
A���� H3=��H1+2��H2 B���� H3=��H1+��H2
C���� H3=��H1��2��H2 D���� H3=��H1����H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ǿ������Ҹ�״̬���ܵ������ ( )
(1)�Ȼ�����Һ�� (2)�Ȼ�粒��壻 (3)ͭ�� (4)ʯī��
(5)����NaOH�� (6) ϡ��� (7)���
A��(1)(2)(5)(6) B��(5)
C��(2)(5) D��(1)(3)(4)(5)(6)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ŵ������ϵ����ϡ����ϡ�������û�з�������Ȼ���ڵļ��ء�20����80�����ѧ�ҽ��г��������о�ʱ��żȻ������ɷ�Ϊ��ɫ�Ĺ���ͭ��(��ѧʽ��BaCuSi2Ox��CuΪ��2��)�������йء����ϡ���˵���в���ȷ���� ( )
A�����ε���ʽ��ʾ�� BaSiO3·CuSiO3 B������������ʽ��ʾ��BaO·CuO·2SiO2
C��������ǿ�ᡢǿ�� D�������ȶ���������ɫ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com