
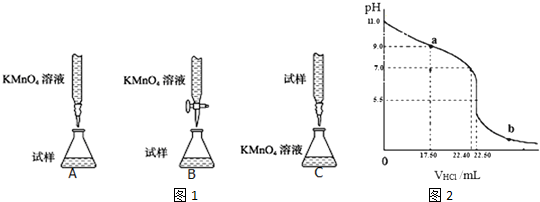
·ÖĪö £Ø1£©¢ŁøßĆĢĖį¼Ų¾ßÓŠĒæŃõ»ÆŠŌ£¬ÄÜŃõ»ÆĻš½ŗ¹Ü£¬²»ÄÜÓĆ¼īŹ½µĪ¶Ø¹Ü£»
¢ŚÅäÖĘČÜŅŗµÄ²½ÖčÓŠ£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ£¬øł¾ŻÅäÖĘ²½ÖčÅŠ¶ĻŹ¹ÓƵÄŅĒĘ÷£¬Č»ŗóČ·¶Ø»¹Č±ÉŁµÄŅĒĘ÷£»
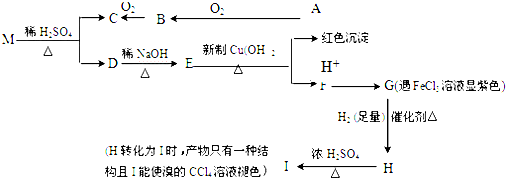
£Ø2£©µ±¼ÓČėĻ”ŃĪĖįµÄĢå»żĪŖ22.40mL£¬pH=7£¬ÓɵēŗÉŹŲŗć£ŗc£ØCl-£©+c£ØOH-£©=c£ØNH4+£©+£ØH+£©Ą“½ā“š£»bµćŃĪĖį¹żĮ棬ČÜŅŗÖŠµÄČÜÖŹĪŖHClŗĶNH4Cl£¬ČÜŅŗĻŌĖįŠŌ£¬øł¾ŻµēĄėŗĶĖ®½āĄ“½ā“š£»
£Ø4£©µ±¼ÓČėĻ”ŃĪĖįµÄĢå»żĪŖ22.40mL£¬pH=7£¬c£ØCl-£©=c£ØNH4+£©£¬øł¾ŻĀČĄė×ÓµÄĪļÖŹµÄĮæ¼ĘĖć£»
£Ø5£©A£®×¶ŠĪĘæÖŠÓŠÉŁĮæÕōĮóĖ®²»Ó°Ļģ°±Ė®µÄĪļÖŹµÄĮ棻
B£®×¶ŠĪĘæ²»ÄÜČóĻ“£»
C£®ĖįŹ½µĪ¶Ø¹ÜĪ“ÓĆŃĪĖįČóĻ“»įµ¼ÖĀŃĪĖįÅضČĘ«µĶ£»
D£®µĪ¶ØÖÕµćŹ±ø©ŹÓ¶ĮŹż»įµ¼ÖĀŃĪĖįĪļÖŹµÄĮæĘ«Š”£®
½ā“š ½ā£ŗ£Ø1£©¢ŁÓĆĖįŠŌKMnO4ČÜŅŗµĪ¶Øij²¹ŃŖ¼Į£¬øßĆĢĖį¼Ų¾ßÓŠĒæŃõ»ÆŠŌ£¬ÄÜŃõ»ÆĻš½ŗ¹Ü£¬²»ÄÜÓĆ¼īŹ½µĪ¶Ø¹Ü£¬ĖłŅŌB·ūŗĻ£»
¹ŹŃ”£ŗB£»
¢ŚÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄĖįŠŌKMnO4ČÜŅŗ250mLµÄ²½ÖčĪŖ£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ²Ł×÷£¬Ņ»°ćÓĆĶŠÅĢĢģĘ½³ĘĮ棬ÓĆŅ©³×Č”ÓĆŅ©Ę·£¬ŌŚÉÕ±ÖŠČܽā£ØæÉÓĆĮæĶ²ĮæČ”Ė®£©£¬ĄäČ“ŗó×ŖŅʵ½250mLČŻĮæĘæÖŠ£¬²¢ÓĆ²£Į§°ōŅżĮ÷£¬Ļ“µÓÉÕ±”¢²£Į§°ō2”«3“Ī£¬½«Ļ“µÓŅŗŅĘČėČŻĮæĘ棬¼ÓĖ®ÖĮŅŗĆę¾ąĄėæĢ¶ČĻß1”«2cmŹ±£¬øÄÓĆ½ŗĶ·µĪ¹ÜµĪ¼Ó£»ĖłŅŌŠčŅŖµÄŅĒĘ÷ĪŖĶŠÅĢĢģĘ½”¢Ņ©³×”¢²£Į§°ō”¢ÉÕ±”¢ĮæĶ²£ØæÉÓĆæɲ»ÓĆ£©£¬250mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü£¬»¹±ŲŠėŹ¹ÓĆµÄ²£Į§ŅĒĘ÷ĪŖ£ŗ½ŗĶ·µĪ¹Ü”¢250mLČŻĮæĘ棻
¹Ź“š°øĪŖ£ŗ½ŗĶ·µĪ¹Ü”¢250mLČŻĮæĘ棻
£Ø2£©µ±¼ÓČėĻ”ŃĪĖįµÄĢå»żĪŖ22.40mLŹ±£¬ČÜŅŗĻŌÖŠŠŌ£¬Ōņc£ØOH-£©=£ØH+£©£¬ÓɵēŗÉŹŲŗćc£ØCl-£©+c£ØOH-£©=c£ØNH4+£©+£ØH+£©æÉÖŖ£¬c£ØCl-£©=c£ØNH4+£©£»bµćŃĪĖį¹żĮ棬ČÜŅŗÖŠµÄČÜÖŹĪŖHClŗĶNH4Cl£¬ČÜŅŗĻŌĖįŠŌ£¬ŌņČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”¹ŲĻµĪŖ£ŗc£ØCl-£©£¾c£ØNH4+£©£¾£ØH+£©£¾c£ØOH-£©£»
¹Ź“š°øĪŖ£ŗ=£»c£ØCl-£©£¾c£ØNH4+£©£¾£ØH+£©£¾c£ØOH-£©£»
£Ø4£©µ±¼ÓČėĻ”ŃĪĖįµÄĢå»żĪŖ22.40mL£¬pH=7£¬c£ØCl-£©=c£ØNH4+£©£¬¶žÕßµÄĪļÖŹµÄĮæĻąµČ£¬ĀČĄė×ÓµÄĪļÖŹµÄĮæĪŖ0.0224L”Į0.0500mol•L-1£¬Ōņļ§øłĄė×ÓµÄĪļÖŹµÄĮ漓°±Ė®µÄĪļÖŹµÄĮæĪŖ0.0224L”Į0.0500mol•L-1£¬ĖłŅŌc£ØNH3•H2O£©=$\frac{0.0224L”Į0.05mol/L}{0.025L}$=0.0448mol/L£»
¹Ź“š°øĪŖ£ŗ0.0448£»
£Ø4£©A£®×¶ŠĪĘæÖŠÓŠÉŁĮæÕōĮóĖ®²»Ó°Ļģ°±Ė®µÄĪļÖŹµÄĮ棬ĖłŅŌ²»Ó°Ļģ²ā¶Ø½į¹ū£¬¹ŹAÕżČ·£»
B£®×¶ŠĪĘæ²»ÄÜČóĻ“£¬ČóĻ“׶ŠĪĘ棬»įŹ¹×¶ŠĪĘæÖŠ°±Ė®µÄĪļÖŹµÄĮæĘ«“ó£¬Ć»ČóĻ“¶Ō²ā¶Ø½į¹ūĪŽÓ°Ļģ£¬¹ŹB“ķĪó£»
C£®ĖįŹ½µĪ¶Ø¹ÜĪ“ÓĆŃĪĖįČóĻ“»įµ¼ÖĀŃĪĖįÅضČĘ«µĶ£¬ŌņŹ¹ÓĆŃĪĖįµÄĢå»żĘ«“ó£¬ĖłŅŌµ¼ÖĀ²ā¶Ø½į¹ūĘ«øߣ¬¹ŹCÕżČ·£»
D£®µĪ¶ØÖÕµćŹ±ø©ŹÓ¶ĮŹż»įµ¼ÖĀŃĪĖįĢå»żĘ«Š”£¬ŌņŃĪĖįĪļÖŹµÄĮæĘ«Š”£¬ĖłŅŌµ¼ÖĀ²ā¶ØĘ«µĶ£¬¹ŹD“ķĪó£®
¹ŹŃ”AC£®
µćĘĄ ±¾Ģāæ¼²éĮĖÖŠŗĶµĪ¶Ø·Ø”¢Ńõ»Æ»¹ŌµĪ¶Ø·Ø£¬²ąÖŲæ¼²éѧɜ·ÖĪö”¢ÅŠ¶Ļ¼°ŹµŃé²Ł×÷ÄÜĮ¦£¬Ć÷Č·µĪ¶ØŌĄķ”¢Čõµē½āÖŹµēĄėµČÖŖŹ¶µćŹĒ½ā±¾Ģā¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 £»¢ŚH”śI£ŗ
£»¢ŚH”śI£ŗ £®
£® £®
£® £®
£® µČ£®£ØČĪŠ“Ņ»ÖÖ£©
µČ£®£ØČĪŠ“Ņ»ÖÖ£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | 115.75mL | B£® | 134.48mL | C£® | 143.75mL | D£® | 156.8mL |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
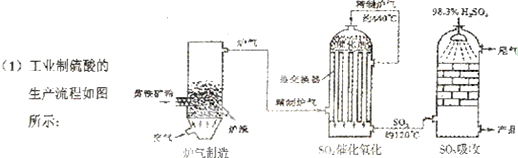
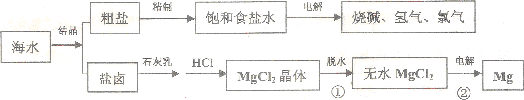
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | µĪ¶ØĒ°µĪ¶Ø¹ÜÖŠÓŠĘųÅŻ£¬µĪ¶ØŗóĻūŹ§ | |
| B£® | ¼īŹ½µĪ¶Ø¹ÜĮæČ”NaOHČÜŅŗŹ±£¬Ī“½ųŠŠČóĻ“²Ł×÷ | |
| C£® | µĪ¶ØŹ±“ļµ½µĪ¶ØÖÕµćŹ±ŃöŹÓ¶ĮŹż | |
| D£® | ׶ŠĪĘæČ”ÓĆNaOH“ż²āŅŗĒ°¼ÓÉŁĮæĖ®Ļ“µÓ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
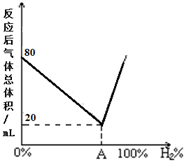 ¢ŁÄ³ĶéĢž·Ö×ÓŹ½ĪŖC6H14£¬ČōøĆĶéĢžæÉÓÉĮ½ÖÖČ²ĢžÓėĒāĘų¼Ó³ÉµĆµ½£¬ŌņøĆĶéĢžµÄ½į¹¹¼ņŹ½ĪŖ£ØCH3£©2CHCH2CH2CH3£®
¢ŁÄ³ĶéĢž·Ö×ÓŹ½ĪŖC6H14£¬ČōøĆĶéĢžæÉÓÉĮ½ÖÖČ²ĢžÓėĒāĘų¼Ó³ÉµĆµ½£¬ŌņøĆĶéĢžµÄ½į¹¹¼ņŹ½ĪŖ£ØCH3£©2CHCH2CH2CH3£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ·Ö×ÓÖŠĖÄøöŌ×Ó¹²Ö±Ļߣ¬ĒŅCĪŖSPŌÓ»Æ | |
| B£® | ·Ö×ÓÖŠN”ŌC¼üµÄ¼ü³¤“óÓŚC-C¼üµÄ¼ü³¤ | |
| C£® | ·Ö×ÓÖŠŗ¬ÓŠ2øö¦Ņ¼üŗĶ4øö¦Š¼ü | |
| D£® | ²»ŗĶĒāŃõ»ÆÄĘČÜŅŗ·¢Éś·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
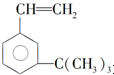 ÓŠČēĻĀŠŌÖŹ£ŗ
ÓŠČēĻĀŠŌÖŹ£ŗ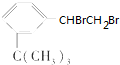 £®
£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com