
 ÓĆ50mL0.50mol/LµÄŃĪĖįÓė50mL0.55mol/LµÄNaOHČÜŅŗŌŚČēĶ¼ĖłŹ¾µÄ×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦£®Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ£®»Ų“šĻĀĮŠĪŹĢā£ŗ
ÓĆ50mL0.50mol/LµÄŃĪĖįÓė50mL0.55mol/LµÄNaOHČÜŅŗŌŚČēĶ¼ĖłŹ¾µÄ×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦£®Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ£®»Ų“šĻĀĮŠĪŹĢā£ŗ·ÖĪö £Ø1£©øł¾ŻÖŠŗĶČČ²ā¶ØŹµŃé³É°ÜµÄ¹Ų¼üŹĒ±£ĪĀ¹¤×÷£»
£Ø2£©øł¾Ż½šŹō°ōŹĒČȵÄĮ¼µ¼Ģ壬Ņד«ČČ£»
£Ø3£©·“Ó¦·Å³öµÄČČĮæŗĶĖłÓĆĖįŅŌ¼°¼īµÄĮæµÄ¶ąÉŁÓŠ¹Ų£¬²¢øł¾ŻÖŠŗĶČȵÄøÅÄīŗĶŹµÖŹĄ“»Ų“š£»
£Ø4£©øł¾ŻĖį¼īÖŠŗĶ·“Ó¦µÄ¶ØŅåŅŖµćŗĶČČ»Æѧ·½³ĢŹ½µÄŹéŠ“ŌŌņ£»
£Ø5£©øł¾ŻČõµē½āÖŹµēĄėĪüČČ·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©ÖŠŗĶČČ²ā¶ØŹµŃé³É°ÜµÄ¹Ų¼üŹĒ±£ĪĀ¹¤×÷£¬“óŠ”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄĖÜĮĻµÄ×÷ÓĆŹĒ¼õÉŁŹµŃé¹ż³ĢÖŠµÄČČĮæĖšŹ§£»
¹Ź“š°øĪŖ£ŗ¼õÉŁŹµŃé¹ż³ĢÖŠµÄČČĮæĖšŹ§£»
£Ø2£©ĢśÖŹ½Į°č°ōµÄµ¼ČČŠŌŗĆ£¬Ņד«ČČ£¬É¢·¢ČČĮ棬»įŹ¹²ā³öµÄĪĀ¶ČĘ«µĶ£¬ĖłµĆÖŠŗĶČČµÄ²ā¶ØÖµ±ČĄķĀŪֵʫµĶ£¬
¹Ź“š°øĪŖ£ŗ²»ÄÜ£»ĢśÖŹ½Į°č°ōŅד«ČČ£¬É¢·¢ČČĮ棬»įŹ¹²ā³öµÄĪĀ¶ČĘ«µĶ£¬ĖłµĆÖŠŗĶČČµÄ²ā¶ØÖµ±ČĄķĀŪֵʫµĶ£»
£Ø3£©·“Ó¦·Å³öµÄČČĮæŗĶĖłÓĆĖįŅŌ¼°¼īµÄĮæµÄ¶ąÉŁÓŠ¹Ų£¬øÄÓĆ60mL0.50mol/LµÄŃĪĖįÓė50mL0.55mol/LµÄNaOHČÜŅŗ½ųŠŠ·“Ó¦£¬ÓėÉĻŹöŹµŃéĻą±Č£¬Éś³ÉĖ®µÄĮæŌö¶ą£¬Ėł·Å³öµÄČČĮæĘ«øߣ¬µ«ŹĒÖŠŗĶČČµÄ¾łŹĒĒæĖįŗĶĒæ¼ī·“Ӧɜ³É1molĖ®Ź±·Å³öµÄČČ£¬ÓėĖį¼īµÄÓĆĮæĪŽ¹Ų£¬ÖŠŗĶČČŹżÖµĻąµČ£»
¹Ź“š°ø£ŗ²»ĻąµČ£»ĻąµČ£»
£Ø4£©ŌŚĻ”ČÜŅŗÖŠ£¬ĒæĖįŗĶĒæ¼ī·¢ÉśÖŠŗĶ·“Ӧɜ³É1mol H2OŹ±£¬·Å³ö57.3kJµÄČČĮ棬·“Ó¦µÄČČ»Æѧ·½³ĢŹ½æÉĪŖ£ŗHCl£Øaq£©+NaOH£Øaq£©=NaCl£Øaq£©+H2O£Øl£©”÷H=-57.3 kJ/mol£»
¹Ź“š°øĪŖ£ŗHCl£Øaq£©+NaOH£Øaq£©=NaCl£Øaq£©+H2O£Øl£©”÷H=-57.3 kJ/mol£®
£Ø5£©“×ĖįŹĒČõµē½āÖŹ£¬Čõµē½āÖŹµēĄė¹ż³ĢÖŠĪüŹÕŅ»²æ·ÖČČĮ棬ĖłŅŌøÄÓĆ60mL0.50mol/LµÄ“×ĖįÓė50mL0.55mol/LµÄNaOHČÜŅŗ½ųŠŠ·“Ó¦£¬ÓėÉĻŹöŹµŃéĻą±Č£¬Ėł·Å³öµÄČČĮæĘ«µĶ£»
¹Ź“š°øĪŖ£ŗĘ«µĶ£»“×ĖįŹĒČõµē½āÖŹ£¬Čõµē½āÖŹµēĄė¹ż³ĢÖŠĪüŹÕŅ»²æ·ÖČČĮ森
µćĘĄ ±¾Ģāæ¼²éÓŠ¹ŲÖŠŗĶČČµÄ²ā¶Ø£¬ÕĘĪÕÖŠŗĶČČµÄ²ā¶ØŌĄķŅŌ¼°Īó²ī·ÖĪöŹĒ½āĢāµÄ¹Ų¼ü£¬ÄŃ¶Č²»“ó£®


 Ó„ÅɽĢøØĻĪ½Ó½Ģ²ÄŗÓ±±½ĢÓż³ö°ęÉēĻµĮŠ“š°ø
Ó„ÅɽĢøØĻĪ½Ó½Ģ²ÄŗÓ±±½ĢÓż³ö°ęÉēĻµĮŠ“š°ø ³õÖŠŹīĘŚĻĪ½ÓĻµĮŠ“š°ø
³õÖŠŹīĘŚĻĪ½ÓĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 800mL0.5mol/LµÄNaClČÜŅŗ | B£® | 100mL0.3mol/LµÄAlCl3ČÜŅŗ | ||
| C£® | 500mL0.3mol/LµÄCaCl2ČÜŅŗ | D£® | 300mL0.3mol/LµÄMgCl2ČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
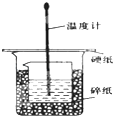 ijŹµŃ銔×éÉč¼ĘÓĆ50mL 1.0mol/LŃĪĖįøś50mL 1.1mol/L ĒāŃõ»ÆÄĘČÜŅŗŌŚČē Ķ¼×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦£®ŌŚ“óÉÕ±µ×²æµęĖéÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬Ź¹·ÅČėµÄŠ”ÉÕ±±æŚÓė“óÉÕ±±æŚĻąĘ½£®Č»ŗóŌŁŌŚ“󔢊”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬“óÉÕ±ÉĻÓĆÅŻÄĖÜĮĻ°å£Ø»ņÓ²Ö½°å£©×÷øĒ°å£¬ŌŚ°åÖŠ¼äæŖĮ½øöŠ”æ×£¬ÕżŗĆŹ¹ĪĀ¶Č¼ĘŗĶ»·ŠĪ²£Į§½Į°č°ōĶعż£®Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
ijŹµŃ銔×éÉč¼ĘÓĆ50mL 1.0mol/LŃĪĖįøś50mL 1.1mol/L ĒāŃõ»ÆÄĘČÜŅŗŌŚČē Ķ¼×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦£®ŌŚ“óÉÕ±µ×²æµęĖéÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬Ź¹·ÅČėµÄŠ”ÉÕ±±æŚÓė“óÉÕ±±æŚĻąĘ½£®Č»ŗóŌŁŌŚ“󔢊”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬“óÉÕ±ÉĻÓĆÅŻÄĖÜĮĻ°å£Ø»ņÓ²Ö½°å£©×÷øĒ°å£¬ŌŚ°åÖŠ¼äæŖĮ½øöŠ”æ×£¬ÕżŗĆŹ¹ĪĀ¶Č¼ĘŗĶ»·ŠĪ²£Į§½Į°č°ōĶعż£®Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ| ŹµŃéŠņŗÅ | ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Č£Øt2£©/”ę | ĪĀ²ī £Øt2-t1£©/”ę | ||
| ŃĪĖį | NaOHČÜŅŗ | Ę½¾łÖµ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  ±ķŹ¾ŌŚĘäĖūĢõ¼ž²»±äŹ±£¬2SO2 £Øg£©+02 £Øg£©?2S03 £Øg£©×Ŗ»Æ¹ŲĻµÖŠ£¬×Ż×ų±ź±ķŹ¾02µÄ×Ŗ»ÆĀŹ | |
| B£® |  ±ķŹ¾ÓĆ0.1mol/L NaOHČÜŅŗ·Ö±šµĪ¶ØµČÅØ¶Č”¢µČĢå»żµÄŃĪĖįŗĶ“×Ėį£¬ĘäÖŠŹµĻßĪŖµĪ¶Ø“×ĖįµÄĒśĻß | |
| C£® |  ±ķŹ¾Ä³ĪüČČ·“Ó¦·Ö±šŌŚÓŠ”¢ĪŽ“߻ƼĮµÄĒéæöĻĀ·“Ó¦¹ż³ĢÖŠµÄÄÜĮæ±ä»Æ | |
| D£® |  ±ķŹ¾³£ĪĀĻĀ£¬Ļ”ŹĶHA”¢HBĮ½ÖÖĖįµÄĻ”ČÜŅŗŹ±£¬ČÜŅŗpHĖę¼ÓĖ®ĮæµÄ±ä»Æ£¬ŌņĖįŠŌHA£¼HB |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ZnĪŖÕż¼«£¬Ģ¼ĪŖøŗ¼« | |
| B£® | øŗ¼«·“Ó¦ĪŖ2NH4++2e-ØT2NH3”ü+H2”ü | |
| C£® | ¹¤×÷Ź±µē×ÓÓÉĢ¼¼«¾ĶāµēĀ·Į÷ĻņŠæ¼« | |
| D£® | ³¤Ź±¼äĮ¬ŠųŹ¹ÓĆŹ±£¬ÄŚ×°µÄŗż×“ĪļæÉÄÜĮ÷³öøÆŹ“µēĘ÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | Fe·Ū£ØĮņ·Ū£© | B£® | Na2CO3·ŪÄ©£ØNaHCO3£© | ||
| C£® | NaCl£Øµā£© | D£® | KMnO4£ØMnO2£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CuSO4 •5H2O”¢“ÅŠŌŃõ»ÆĪļ¶¼ŹĒ“æ¾»Īļ | |
| B£® | Ņ»ÖÖĪļÖŹ²»ŹĒµē½āÖŹ¾ĶŹĒ·Ēµē½āÖŹ | |
| C£® | Ļ”ĮņĖį”¢NaClČÜŅŗŹĒŹµŃéŹŅ³£¼ūµÄµē½āÖŹ | |
| D£® | ¶žŃõ»ÆĮņæɹć·ŗÓĆÓŚŹ³Ę·µÄŌö°× |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| Al£ØOH£©3 | Ga£ØOH£©3 | |
| ĖįŹ½µēĄė³£ŹżKa | 2”Į10-11 | 1”Į10-7 |
| ¼īŹ½µēĄė³£ŹżKb | 1.3”Į10-33 | 1.4”Į10-34 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com