
(1)�״���һ����Ҫ�Ļ�����Ʒ�������ü״��������Ʊ���ȩ����ȩ����̬�״�ת����������ϵ��ͼ��ʾ��
��Ӧ�����е�������ϵ
�ټ״�������ת��Ϊ��ȩ�ķ�Ӧ��________(����ȡ����ȡ�)��Ӧ��
�ڹ��̢�����̢�ķ�Ӧ���Ƿ���ͬ��____________ԭ����__________________________________��
��д���״�������ת��Ϊ��ȩ���Ȼ�ѧ��Ӧ����ʽ________________________________��
(2)��֪����CH3OH(g)��H2O(g)=CO2(g)��3H2(g)����H����49.0 kJ��mol��1
��CH3OH(g)�� O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
����˵����ȷ����________��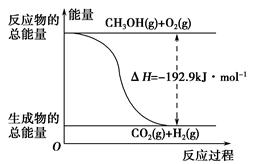
| A��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| B���ٷ�Ӧ�У���Ӧ�������������������������� |
C�����ݢ���֪��Ӧ��CH3OH(l)�� O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 |
| D����Ӧ�ڵ������仯��ͼ��ʾ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ǰ��ʱ��ϯ���ҹ����������������ǵ�������������˼����Ӱ�죬��ͳ���ҹ����ֳ���������ռȫ��һ�룬����������PM2.5ϸ���Ӱ���(NH4)2SO4��NH4NO3������������л������P�ﳾ�ȡ�
��1���л�������IJ�����Ҫ�����ڲ���ȫȼ�յ��µ�����Ȼ�ѧ����ʽ���£�
��C(s)��O2(g)=CO2(g)����H1����94kJ��mol��1��
��C8H16(l)+12O2(g)=8CO2(g)+8H2O(l) ��H2����1124kJ��mol��1
��C8H16(l)+4O2=8C��g��+8H2O��l����H3�� kJ��mol��1
��2�����������ѿɹ��ӷ����л���Ⱦ�VOCs��������ˮ�������ڣ�������ϩ���ⷴӦΪ��C2HCl3+2O2��2CO2+HCl+Cl2�������㹻���Ľ�����β����ʵ���Ҽ���������������ļ����ǣ� ��ͨ�������Ƿ��ֻ��ж��ָ��������֮һΪ��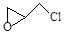 ������л���˴Ź��������� �� ���塣
������л���˴Ź��������� �� ���塣
��֪��Cu(OH)2�Ƕ�Ԫ��������ᣨH3PO3���Ƕ�Ԫ���ᣬ��NaOH��Һ��Ӧ������Na2HPO3��
��3����ͭ����Һ��Cu2������ˮ�ⷴӦ�����ӷ���ʽΪ____���÷�Ӧ��ƽ�ⳣ��Ϊ____������֪��25��ʱ��Ksp[Cu(OH)2]��2.0��10��20mol3/L3��
��4�����Na2HPO3��Һ�ɵõ������ᣬװ����ͼ��˵������Ĥֻ����������ͨ������Ĥֻ����������ͨ����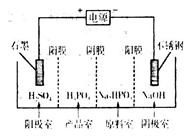
�������ĵ缫��ӦʽΪ____________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CH4��H2��C�������ʵ���Դ���ʣ�����ȼ�յ��Ȼ�ѧ����ʽΪ��
��CH4(g)��2O2(g)=CO2(g)��2H2O(l)����H����890.3 kJ��mol��1��
��2H2(g)��O2(g)=2H2O(l)����H����571.6 kJ��mol��1��
��C(s)��O2(g)=CO2(g)����H����393.5 kJ��mol��1��
(1)����д���һ�ּ���ϸ������������øʹ������O2���ò���������������ϸ��ʹ1 mol��������CO2������Һ̬ˮ���ų�������________(���������������)890.3 kJ��
(2)������CO2�����ںϳɺϳ���(��Ҫ�ɷ���һ����̼������)��CH4��CO2=2CO��2H2��1 g CH4��ȫ��Ӧ���ͷ�15.46 kJ����������
����ͼ�ܱ�ʾ�÷�Ӧ�����������仯����________(����ĸ)��
���������ʵ�����Ϊ1 mol��CH4��CO2����ij�����ܱ������У���ϵ�ų�����������ʱ��ı仯��ͼ��ʾ����CH4��ת����Ϊ________��
(3)C(s)��H2(g)����Ӧ������C(s)��2H2(g)=CH4(g)�ķ�Ӧ����ֱ�Ӳ�������ͨ��������Ӧ�������C(s)��2H2(g)=CH4(g)�ķ�Ӧ�Ȧ�H��________��
(4)Ŀǰ���������������ʵ��о���ȼ���о����ص㣬���й��������������ʵ��о������п��е���________(����ĸ)��
| A��Ѱ�����ʴ�����ʹCO2��H2O��Ӧ����CH4��O2�����ų����� |
| B��Ѱ�����ʴ������ڳ��³�ѹ��ʹCO2�ֽ�����̼��O2 |
| C��Ѱ�����ʴ���������̫����ʹ�����е�CO2�뺣���ɵ�CH4�ϳɺϳ���(CO��H2) |
| D������̬̼�ϳ�ΪC60����C60��Ϊȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����֪�� C(s)+O2(g)=CO2(g) ��H1����393.5 kJ/mol
C(s)+H2O(g)=CO(g)+H2(g) ��H2����131.3 kJ/mol
��ӦCO(g)+H2(g) +O2(g)= H2O(g)+CO2(g)����H= ____ ___kJ/mol��
��2����һ���ݵ��ܱ������У���CO��H2�ϳɼ״���CO(g)+2H2(g) CH3OH(g) ��H
CH3OH(g) ��H
���������β���˵���÷�Ӧ�Ѵﵽƽ��״̬����_______������ţ���
A��ÿ����1 mol CO��ͬʱ����2molH2
B��������������ʵ�������
C������CH3OH������������CO���������
D��CH3OH��CO��H2��Ũ�ȶ����ٷ����仯
��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��A��B�����ƽ�ⳣ��K(A)_______K(B)�����������=������,��ͬ������ͼ�жϦ�H _____0��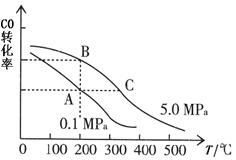
��ij�¶��£���2.0 mol CO��6.0 molH2����2 L���ܱ������У���ַ�Ӧ�ﵽƽ��ʱ���c(CO)="0.25" mol/L����CO��ת����= �����¶��µ�ƽ�ⳣ��K= ��������λ��Ч���֣���
��3�������¶�650���������ȼ�ϵ�أ���ú̿����CO��H2����������Ӧ�������CO2�Ļ������Ϊ������Ӧ����������缫����һ��������Li2CO3��Na2CO3���۵�����������ʡ������ĵ缫��ӦʽΪ��CO+H2��4e-+2CO32-=3CO2+H2O����õ�ص�������ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)��֪��H2(g)��1/2O2(g)=H2O(l)����H����285.8 kJ��mol��1
H2(g)=H2(l)����H����0.92 kJ��mol��1
O2(g)=O2(l)����H����6.84 kJ��mol��1
H2O(l)=H2O(g)����H����44.0 kJ��mol��1
��д��Һ���Һ����Ӧ������̬ˮ���Ȼ�ѧ����ʽ��__________________________
�������ҺΪKOH��Һ������ȼ�ϵ�أ��为���ĵ缫��ӦʽΪ____________________________________��
(2)��ͼ��ʾ373 Kʱ����ӦA(g)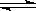 2B(g)��ǰ110 s�ڵķ�Ӧ���̡�
2B(g)��ǰ110 s�ڵķ�Ӧ���̡�
�ٴ˷�Ӧ��ƽ�ⳣ������ʽK��________��
��373 Kʱ��Ӧ���е�70 sʱ���ı������������________����Ӧ���е�90 sʱ���ı������������________��
| A��������� | B������������� | C�������¶� | D������A��Ũ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)(�㶫)����ʯ[��Ҫ�ɷ�Ca5(PO4)3F]�ڸ������Ʊ�����(P4)���Ȼ�ѧ����ʽΪ��4Ca5(PO4)3F(s)��21SiO2(s)��30C(s)===3P4(g)��20CaSiO3(s)��30CO(g)��SiF4(g)����H
��������Ӧ�У����������������________��
����֪��ͬ�����£�
4Ca5(PO4)3F(s)��3SiO2(s)===6Ca3(PO4)2(s)��2CaSiO3(s)��SiF4(g)����H1
2Ca3(PO4)2(s)��10C(s)===P4(g)��6CaO(s)��10CO(g)����H2
SiO2(s)��CaO(s)===CaSiO3(s)����H3
�æ�H1����H2�ͦ�H3��ʾ��H����H��____________��
(2)(����)��H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
��Cu(s)��2H��(aq)===Cu2��(aq)��H2(g) ��H1����64.39 kJ��mol��1
��2H2O2(l)===2H2O(l)��O2(g) ��H2����196.46 kJ��mol��1
��H2(g)�� O2(g)===H2O(l) ��H3����285.84 kJ��mol��1
O2(g)===H2O(l) ��H3����285.84 kJ��mol��1
��H2SO4��Һ�У�Cu��H2O2��Ӧ����Cu2����H2O���Ȼ�ѧ����ʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ĿǰΪֹ���ɻ�ѧ��ת��Ϊ���ܻ������Ȼ������ʹ������Ҫ����Դ��
��1����ѧ��Ӧ�зų������ܣ��ʱ䣬��H���뷴Ӧ����������ڷ�Ӧ�����жϼ����γ��¼����������պͷų������Ĵ�С�йء�
��֪��H2��g����Cl2��g��=2HCl��g�� ��H����185 kJ/mol������1 mol H��H�����յ�����Ϊ436 kJ������1 mol Cl��Cl�����յ�����Ϊ247 kJ�����γ�1 mol H��Cl���ų�������Ϊ ��
��2��ȼ��ȼ�ս��������Ļ�ѧ��ת��Ϊ��������Ҫ�����ܡ���֪��
��CH4��g����2O2��g��=CO2��g����2H2O��l�� ��H����890��3 kJ��mol-1
��C��s,ʯī����O2��g��=CO2��g�� ��H����393��5 kJ��mol��1
��2H2��g����O2��g��=2H2O��l�� ��H����571��6 kJ��mol-1
��״����22��4 L�����ͼ���Ļ�������������������г��ȼ�շ�Ӧ�ų�588��05 kJ��������ԭ��������������������� ���������������Ȼ�ѧ����ʽ������C��s,ʯī����2H2��g��=CH4��g���ķ�Ӧ�Ȧ�HΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ѣ�DME������Ϊ��21���͵����ȼ�ϡ����ɺϳ����Ʊ������ѵ���Ҫԭ�����£�
�� CO(g)+2H2(g)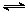 CH3OH(g) ��H 1=��90.7 kJ��mol-1
CH3OH(g) ��H 1=��90.7 kJ��mol-1
�� 2CH3OH(g)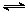 CH3OCH3(g)+H2O(g) ��H 2=��23.5 kJ��mol-1
CH3OCH3(g)+H2O(g) ��H 2=��23.5 kJ��mol-1
�� CO(g)+H2O(g)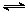 CO2(g)+H2(g) ��H 3=��41.2kJ��mol-1
CO2(g)+H2(g) ��H 3=��41.2kJ��mol-1
�ش��������⣺
��1����Ӧ3H2(g)��3CO(g) CH3OCH3(g)��CO2(g)�ġ�H�� kJ��mol-1��
CH3OCH3(g)��CO2(g)�ġ�H�� kJ��mol-1��
��2�����д�ʩ�У������CH3OCH3���ʵ��� ��
A��ʹ�ù�����CO B�������¶� C������ѹǿ
��3����Ӧ�������CH3OCH3�IJ��ʣ�ԭ���� ��
��4�����ϳ�����n(H2)/n(CO)=2ͨ��1 L�ķ�Ӧ���У�һ�������·�����Ӧ��
4H2(g)+2CO(g) 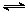 CH3OCH3(g)+H2O(g) ��H����CO��ƽ��ת�������¶ȡ�ѹǿ�仯��ϵ��ͼ1��ʾ������˵����ȷ���� ��
CH3OCH3(g)+H2O(g) ��H����CO��ƽ��ת�������¶ȡ�ѹǿ�仯��ϵ��ͼ1��ʾ������˵����ȷ���� ��
A����H <0
B��P1<P2<P3
C������P3��316��ʱ����ʼʱn(H2)/n(CO)=3����ﵽƽ��ʱ��COת����С��50��[
��5������һ�����͵Ĵ�������Ҫ�ɷ���Cu-Mn�ĺϽ𣩣�����CO��H2�Ʊ������ѡ��۲�ͼ2�ش����⡣������n(Mn)/n(Cu)ԼΪ ʱ�������ڶ����ѵĺϳɡ�
��6��ͼ3Ϊ��ɫ��Դ��������ȼ�ϵ�ء��Ĺ���ԭ��ʾ��ͼ��a�缫�ĵ缫��ӦʽΪ ��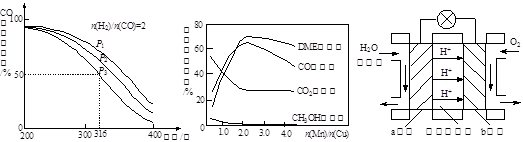
ͼ1 ͼ2 ͼ3
��7���״�Һ����ˮ���ƶ����ѵ�ԭ���ǣ�CH3OH +H2SO4��CH3HSO4+H2O��
CH3 HSO4+CH3OH��CH3OCH3+H2SO4����ϳ����Ʊ������ѱȽϣ��ù��յ��ŵ��Ƿ�Ӧ�¶ȵͣ�ת���ʸߣ���ȱ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ǵ����Ϻ����ḻ��һ��Ԫ�أ��䵥�ʼ��������ڹ�ũҵ������������������Ҫ���á�
��1��һ���¶��£���1L�ݻ��㶨���ܱ������г���2 mol N2��8molH2��������Ӧ��10min��ƽ�⣬��ð�����Ũ��Ϊ0��4 mol��L��1����ʱ������ת����Ϊ________��������߰����IJ��ʣ����ݻ�ѧƽ���ƶ�ԭ������������Ľ���______________��д��һ�����ɣ���
��2����ͼ��1mol NO2��g����1mol CO��g����Ӧ����lmol CO2��g����1 mol NO��g�������������仯ʾ��ͼ����д���÷�Ӧ���Ȼ�ѧ����ʽ_____________________��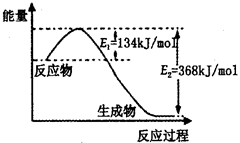
��3�����ݻ��㶨���ܱ������У��������·�Ӧ��N2��g����3H2��g��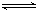 2NH3��g����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3��g����H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���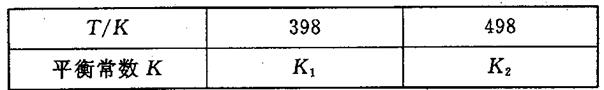
�ٸ÷�Ӧ��ƽ�ⳣ������ʽ��K��_____________��
�����ж�K1__________K2����д��������������������
��NH3��g��ȼ�յķ���ʽΪ��4NH3��g����7O2��g����4NO2��g����6H2O��l������֪��
��2H2��g����O2��g�� 2H2O��l�� ��H����483��6 kJ��mol
2H2O��l�� ��H����483��6 kJ��mol
��N2��g����2O2��g�� 2NO2��g�� ��H����67��8 kJ��mol
2NO2��g�� ��H����67��8 kJ��mol
��N2��g����3H2��g�� 2NH3��g�� ��H����92��0 kJ��mol
2NH3��g�� ��H����92��0 kJ��mol
�����NH3��g����ȼ����________kJ��mol��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com