��2011?�Ͼ�ģ�⣩ij�ֺ�������FeCl
2���ʵ�FeCl
3��Ʒ����Ҫ�ⶨ������Ԫ�صĺ�����ʵ��������²�����У�
1��ȷ����m g��Ʒ��2��3g����
2������Ʒ�м���10mL 5mol/L�����ᣬ�ټ�������ˮ�����Ƴ�250mL��Һ��
3����ȡ25mL����������õ���Һ������3mL��ˮ������ʹ֮��ȫ��Ӧ��
4������Ѹ�ټ���Ũ��Ϊ10%�İ�ˮ����������ֽ��裬ʹ֮��ȫ������
5�����ˣ�������ϴ�ӡ����ա���ȴ�������������������أ�
���������������ش�
��1��������ǰ��������ƽ��ָ��ƫ��������������ʱ��ָ��պ��ڱ�ߵ��м䣬��������Ʒ������
B
B
��
A����mg�� B����mg�� C��ǡ��Ϊmg
��2���ܽ���ƷʱҪ�������ᣬԭ����
����Fe2+��Fe3+��ˮ��
����Fe2+��Fe3+��ˮ��
��
��3������250mL��Һʱ������250mL������ƿ���ձ��⣬�����õ��IJ���������
����������ͷ�ι�
����������ͷ�ι�
��
��4��������ˮʱ������Ӧ�����ӷ���ʽ��
2Fe2++Br2=2Fe3++2Br-
2Fe2++Br2=2Fe3++2Br-
��
��5������������ΪW
1 g�����������պ�������������W
2g������Ʒ����Ԫ�ص�����������
��
��6����������250mL��Һʱ�����õ�����ƿû��ϴ�ɾ�����������������ʱ�����ջ�ʹ��Ԫ�صIJⶨ�������ƫ�ߡ�����ƫ�͡����䡱������NaCl
����
����
��AlCl
3ƫ��
ƫ��
��



 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
 ��2011?�Ͼ�ģ�⣩�����£���10mL 0.1mol?L-1NaOH��Һ����μ���0.1mol?L-1������Һ�����õζ�������ͼ��ʾ������˵����ȷ���ǣ�������
��2011?�Ͼ�ģ�⣩�����£���10mL 0.1mol?L-1NaOH��Һ����μ���0.1mol?L-1������Һ�����õζ�������ͼ��ʾ������˵����ȷ���ǣ�������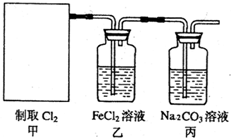 ��2011?�Ͼ�ģ�⣩������һ����Ҫ�Ļ���ԭ�ϣ�
��2011?�Ͼ�ģ�⣩������һ����Ҫ�Ļ���ԭ�ϣ�