
ij��Һ���ܺ���Cl����SO42����CO32����NH4+��Fe3+��Fe2+��Al3+ ��Na+��ijͬѧΪ��ȷ����ɷ֣�ȡ������Һ����Ʋ����������ʵ�飺
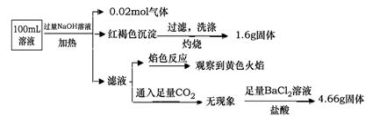
�ɴ˿�֪ԭ��Һ�У� ��
A��ԭ��Һ��c��Fe3+��=0.2 mol��L-1
B����Һ��������4�����Ӵ��ڣ�����Cl��һ�����ڣ���c��Cl������0.2 mol��L-1
C��SO42����NH4+ ��Na+һ�����ڣ�CO32����Al3+һ��������
D��Ҫȷ��ԭ��Һ���Ƿ���Fe2+,�����Ϊ��ȡ����ԭ��Һ���Թ���,�����������Ը��������Һ������Һ�Ϻ�ɫ��ȥ����֤��ԭ��Һ����Fe2+
 ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017���½�����������Ŷ��и����ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪���������������·�Ӧ��2Cu+=Cu2++Cu�����ڷ�Ӧ�¶Ȳ�ͬ����������ԭ����ͭʱ�����ܲ���Cu��Cu2O�����߶��Ǻ�ɫ���塣һλͬѧ��ij����������ԭ����ͭʵ�����õĺ�ɫ�������������֤��ʵ�������ʵ�������¼���£�
�����Լ� | ϡ���� | Ũ������� | ϡ���� | Ũ���� |
ʵ������ | ��ɫ�������ɫ��Һ | ��ɫ���� | ��ɫ�������ɫ��Һ | ����ɫ�������ɫ��Һ |
�ɴ��Ƴ�����������ԭ����ͭʵ��IJ�����( )
A��Cu B��Cu2O C��һ����Cu��������Cu2O D��һ����Cu2O������Cu
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����ϵ�һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
25��ʱ��ȡŨ�Ⱦ�Ϊ0.1 mol��L��1�Ĵ�����Һ�Ͱ�ˮ��Һ��20 mL���ֱ���0.1 mol��L��1NaOH��Һ��0.1 mol��L��1��������к͵ζ����ζ�������pH��μ���Һ������仯��ϵ��ͼ��ʾ������˵����ȷ���ǣ� ��
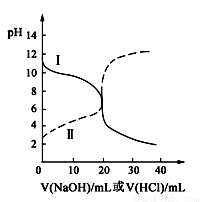
A�����ߢμ���Һ��10 mLʱ��
c(CH3COO��)��c(Na��)��c(H��)��c(OH��)
B�����ߢμ���Һ��20 mLʱ��
c(Cl��)=c(NH4��)��c(H��)=c(OH��)
C�����ߢμ���Һ��10 mL��20 mL֮����ڣ�
c(NH4��)��c(Cl��)��c(OH��)��c(H��)
D�����ߢμ���Һ��10 mLʱ��
c(CH3COO��)��c(CH3COOH)��2[c(H��)��c(OH��)]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�Ͳ��и߶���ѧ�ڿ�ѧ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
���и����е����ӣ�������Һ�д����������
A��K+��Cu2+��Cl����OH�� B��Mg2+��SO42����K+��Cl��
C��Na+��H+��NO3����CO32�� D��Ba2����Na+��OH����SO42��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ��«������УЭ����߶����ڳ����Ի�ѧ���������棩 ���ͣ��ƶ���
ij��ɫ��Һ����Na����Ag����Ba2����Al3����AlO2-��MnO4-��CO32-��SO42-�е���������ɣ�ȡ����Һ��������ʵ�飺
(A)ȡ������Һ������������ᣬ���������ɣ����õ���Һ��
(B)��(A)������Һ���ټ������̼�������Һ�����������ɣ�ͬʱ������ɫ�����ף�
(C)��(B)������Һ�м������Ba(OH)2��Һ��Ҳ���������ɣ����а�ɫ������������
��������ʵ��ش��������⡣
��1����Һ��һ�������ڵ�������_______________________��
��2��һ�����ڵ�������_______________________________��
д���������������ӷ���ʽ��____________________________��
��3���жϳ����ҳɷֵķ�����______________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ��«������УЭ����߶����ڳ����Ի�ѧ���������棩 ���ͣ�ѡ����
���ֶ���������Ԫ�� A��B��C��D��E ��ԭ���������ε�����A2������ɫȼ�ϣ�C�������ﳣ���ڲ�����������DԪ��ԭ�ӵĺ˵������ͬ������һ����Ԫ�ص�2����B��C Ϊͬ����Ԫ�أ�B��D ԭ������������֮�͵���E�������������� ������������������˵������ȷ����( )
A. ����Ԫ����BԪ�صĽ�������ǿ
B. Ԫ��D��E�ֱ���Ԫ��A�γɻ�������ȶ��ԣ�A2D>AE
C. Ԫ��C��D��E������������Ӧˮ��������Եݼ�
D. Ԫ��B��D��E�ļ����Ӱ뾶��СΪ��B>D>E
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ��«������УЭ����߶����ڳ����Ի�ѧ���������棩 ���ͣ�ѡ����
���г��ӵIJ�����������ȷ���ǣ� ��
A. NO����������NO2��ͨ��װ��ˮ��ϴ��ƿ
B. SO2�л�������HCl���壺ͨ������NaHSO3��Һϴ��
C. O2����������CO2��ͨ��װ�м�ʯ�ҵ�U�ι�
D. ʳ������������NaHCO3���ӹ������ռ���Һ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ�����и߶���9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
һ������ȼ�ϵ�أ�һ��ͨ���������һ��ͨ�붡�����壻������Dz���������?Y2O3?�������?ZrO2?���壬������״̬���ܴ���O2?�����жԸ�ȼ�ϵ��˵����ȷ����
A�������ڵ�����У�O2?�ɸ�����������
B����ص��ܷ�Ӧ�ǣ�2C4H10��13O2 ? 8CO2��10H2O
C��ͨ�������һ�����������缫��ӦΪ��O2��4e?��2H2O��4OH?
D��ͨ�붡���һ�����������缫��ӦΪ��2C4H10��26e?��13O2?��4CO2��5H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶���ѧ��9�µ��л�ѧ�Ծ��������棩 ���ͣ�ѡ����
ȫ������Զ��Բ������缫���ڵ������Һ�з����ĵ���ܷ�ӦΪ��VO2������ɫ����H2O��V3������ɫ�� VO2������ɫ����V2������ɫ����2H�� ����˵������ģ� ��
VO2������ɫ����V2������ɫ����2H�� ����˵������ģ� ��
A.���ʱ����Ӧÿ����2molH��ʱ����ת�Ƶ����ʵ���Ϊ2mol
B.�ŵ�����У�����������Һ�����Լ���
C.�ŵ�ʱ��������ӦΪVO2++2H����e����VO2����H2O
D.���ʱ������������Һ����ɫ��Ϊ��ɫ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com