
��������Ҫ������Դ��������Ϊ�������õĹؼ��������ǵ�ǰ��ע���ȵ�֮һ��
(1)���������ȼ�ϣ���ȼ�ղ���Ϊ________��
(2)NaBH4��һ����Ҫ�Ĵ������壬����ˮ��Ӧ�õ�NaBO2���ҷ�Ӧǰ��B�Ļ��ϼ۲��䣬�÷�Ӧ�Ļ�ѧ����ʽΪ__________________________________________����Ӧ����1 mol NaBH4ʱת�Ƶĵ�����ĿΪ________��
 (3)����ɽ����л�������û�����ͱ�֮��Ŀ��淴Ӧ��ʵ������ͼ��⣺
(3)����ɽ����л�������û�����ͱ�֮��Ŀ��淴Ӧ��ʵ������ͼ��⣺
 (g)
(g)  (g)��3H2(g)��
(g)��3H2(g)��
��ij�¶��£�������ܱ������м��뻷���飬����ʼŨ��Ϊa mol��L��1��ƽ��ʱ����Ũ��Ϊb mol��L��1���÷�Ӧ��ƽ�ⳣ��K��________��
(4)һ�������£���ͼ��ʾװ�ÿ�ʵ���л���ĵ绯ѧ����(���������л���)��

�ٵ����е����ƶ�����Ϊ________��(��A��D��ʾ)
������Ŀ�����ĵ缫��ӦʽΪ__________________��
�۸ô���װ�õĵ���Ч�ʦǣ�____________________��(�ǣ�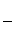 ��100%������������С�����1λ)
��100%������������С�����1λ)
[��]��(1)H2O
(2)NaBH4��2H2O===NaBO2��4H2����
4NA��2.408��1024
(3) mol3��L��3
mol3��L��3
(4)��A��D����C6H6��6H����6e��===C6H12����64.3%[����] (2)BԪ�ط�Ӧǰ�ϼ۲��䣬������Ӧ��������NaBO2��֪����ӦǰNaBH4��B�Ļ��ϼ�Ҳ�ǣ�3�ۣ���HΪ��1�ۣ�ˮ����Ԫ�ػ��ϼ�Ϊ��1�ۣ��������з�Ӧ����������������Ԫ�ػ��ϼۿ�ȷ��ʧȥ��������(3)���ݻ�ѧ����ʽ��֪������H2Ϊ3b mol/L�����Ļ�����b mol/L����ƽ��ʱ������Ϊ(a��b) mol/L��������ʽȷ��ƽ�ⳣ����(4)�ٱ����ɻ������ǵ��ⷴӦ��Ϊ��ԭ��Ӧ�����缫D���������缫E�������������е����ƶ�����A��D����Ŀ������ǻ����飬������ͨ�����ӽ���Ĥ�������ƶ�����缫��ӦʽΪC6H6��6H����6e��===C6H12������������2.8 mol���壬��������OH���������ŵ������������ת�Ƶ���11.2 mol�����������ı������ʵ�����x mol��ͬʱ����x mol�����飬���缫��Ӧʽ��֪ת�Ƶĵ���Ϊ6x mol������ݵ����غ�֪��ͬʱ����������(11.2 mol��6x mol)��2��5.6 mol��3x mol�����ݻ������ɷֿ���ʽ 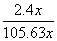 ��10%�����x��1.2����˴���װ�õĵ���Ч��Ϊ
��10%�����x��1.2����˴���װ�õĵ���Ч��Ϊ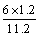 ��64.3%��
��64.3%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
V L Fe2(SO4)3��Һ�к���a g SO ��ȡ����Һ0.5V L����ˮϡ����2V L����ϡ�ͺ���Һ��Fe3�������ʵ�����Ũ��Ϊ(����)
��ȡ����Һ0.5V L����ˮϡ����2V L����ϡ�ͺ���Һ��Fe3�������ʵ�����Ũ��Ϊ(����)
A. mol/L B.
mol/L B. mol/L
mol/L
C. mol/L D.
mol/L D. mol/L
mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���������������в���ȷ����(����)
�����������������ˮ��Ӧ������Ӧ���ᡡ�ڷǽ���������϶�������������ۼ���������϶��ǽ���������ܽ��������ﶼ�Ǽ���������ݲ��ܸ��ᷴӦ��������һ���ܸ��Ӧ
A���٢ڢܢ� B���٢ڢۢܢ�
C���ڢۢ� D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȷ��ʾ���з�Ӧ�����ӷ�Ӧ����ʽΪ(����)
A��NH4HCO3���ڹ�����ŨKOH��Һ�У�
NH �� HCO
�� HCO ��2OH��=== CO
��2OH��=== CO �� NH3����2H2O
�� NH3����2H2O
B����������Һ�еμ�Ba(OH)2��Һ��ǡ��ʹSO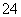 ������ȫ��2Al3����3SO
������ȫ��2Al3����3SO ��3Ba2����
��3Ba2����
6OH��===2Al(OH)3����3BaSO4��
C����FeBr2��Һ��ͨ������������2Fe2����4Br����3Cl2===2Fe3����2Br2��6Cl��
D�������ȥˮ����2H����CaCO3===Ca2���� CO2���� H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ӱ�����ǵ������뽡����ij�����������п��ܺ������¿����������ӣ�Na����NH ��Mg2����Al3����SO
��Mg2����Al3����SO ��NO
��NO ��Cl����ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ�������������Һ����Ʋ����������ʵ�飺
��Cl����ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ�������������Һ����Ʋ����������ʵ�飺
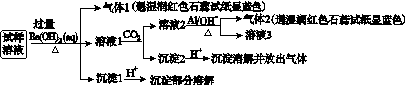
��֪��3NO ��8Al��5OH����2H2O
��8Al��5OH����2H2O 3NH3����8AlO
3NH3����8AlO
�������ϵ�ʵ�����������ͬѧ�ó��Ľ��۲���ȷ����(����)
A�������п϶�����NH ��Mg2����SO
��Mg2����SO ��NO
��NO
B��������һ������Al3��
C�������п��ܴ���Na����Cl��
D���������п��ܴ���NaNO3��NH4Cl��MgSO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�弰�仯����㷺Ӧ����ҽҩ��ũҩ����ά��������ȼ���ȣ��ش��������⣺
(1)��ˮ��������У���Ũ���ĺ�ˮ��ͨ��________�������е�Br�����������ÿ��������壻Ȼ����̼������Һ�����壬���绯ΪBr����BrO �������ӷ���ʽΪ__________________________________________��
�������ӷ���ʽΪ__________________________________________��
(2)���������Թ��ۼ�����γ�BrCl��BrCl�����У�________�������ԡ�BrCl��ˮ������Ӧ�Ļ�ѧ����ʽΪ____________________________________________��
(3)CuBr2�ֽ���Ȼ�ѧ����ʽΪ��
2CuBr2(s)===2CuBr(s)�� Br2(g)
��H����105.4 kJ/mol
���ܱ������н�����CuBr2��487 K�¼��ȷֽ⣬ƽ��ʱp(Br2)Ϊ4.66��103 Pa��
���練Ӧ��ϵ��������䣬��߷�Ӧ�¶ȣ���p(Br2)����________(��������䡱��С��)��
���練Ӧ�¶Ȳ��䣬����Ӧ��ϵ���������һ������p(Br2)�ı仯��ΧΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���

��.�Ʊ�Na2S2O3��5H2O
��Ӧԭ����Na2SO3(aq)��S(s) Na2S2O3(aq)
Na2S2O3(aq)
ʵ�鲽�裺
�ٳ�ȡ15 g Na2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5 g��ϸ����ۣ���3 mL�Ҵ���ʪ������������Һ�С�
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60 min��
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O3��5H2O�������ˡ�ϴ�ӡ�����õ���Ʒ��
�ش����⣺
(1)����ڷ�Ӧǰ���Ҵ���ʪ��Ŀ����__________________________��
(2)����a��������________����������____________________��
(3)��Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������______________�������Ƿ���ڸ����ʵķ�����____________________________��
(4)��ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ�Ӧ����ʽ��ʾ��ԭ��________________________________________________________________________
________________________________________________________________________��
��.�ⶨ��Ʒ����
ȷ��ȡW g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 mol��L��1��ı���Һ�ζ���
��Ӧԭ��Ϊ2S2O ��I2===S4O
��I2===S4O ��2I��
��2I��
(5)�ζ����յ�ʱ����Һ��ɫ�ı仯��____________________________________________��
(6)�ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ__________mL����Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)______________��

��.Na2S2O3��Ӧ��
(7)Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO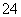 �����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ҵ����͡�����������������������Ҵ���϶��ɵ�һ��ȼ�ϡ������й���������ȷ����(����)
A������ʹ���Ҵ������ܼ����к�������ŷ�
B����ҵ�ϳ����ѻ��ķ���������͵IJ���������
C���Ҵ������Ƕ������Ļ����
D�������ס��������Ϳ����Ƶ��Ҵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D��E����������������ɵĿ����Ի����������������ʵ�������(���Ӳ����ظ����)��
| ������ | Na����Mg2����Al3����Ba2����Fe3�� |
| ������ | OH����Cl����CO |
�ֱ�ȡ���ǽ���ʵ�飬������£�
��A��Һ��D��Һ��ϣ�û����������
�ڵ�B��Һ���뵽D��Һ��ʱ���г�����������������B��Һ������ȫ����ʧ��
�۽�E��Һ���ȣ��г������ɡ�
�ݴ��ƶϣ�A________��B________��C________��
D________��E_____ ___��
___��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com