
| ����ϸ�� |
| -2 |
| O |
| 18 |
| 14 |
| n |
| V |
| -2 |
| O |
| -2 |
| O |
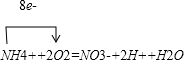
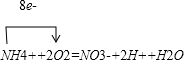 ��
��| 18 |
| 14 |
| 64g |
| 18g |
| 18 |
| 14 |
| 19.2g |
| 64g/mol |
| 0.2mol |
| 0.1L |


 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?�Ϻ�ģ�⣩����ɫ��ѧʵ�顱�����ã�ij��ѧ��ʦΪ������������Ʒ�Ӧ�����������װ��������������صĿα�ʵ�飮ʵ�����������������Ӧ��װ�ÿ�����ͼ�Ľ�����һ���������������������������������ڷ�һ��ƶ�����Ľ����ƣ�������ú�ͣ���������β����һ�Ž���NaOH��Һ�������ȸ���Ԥ�ȣ��������ڳ�Բ��ʱ������ͨ�����������ɼ����Ż�ȼ�գ����ɴ������̣���������������ǣ�������
��2012?�Ϻ�ģ�⣩����ɫ��ѧʵ�顱�����ã�ij��ѧ��ʦΪ������������Ʒ�Ӧ�����������װ��������������صĿα�ʵ�飮ʵ�����������������Ӧ��װ�ÿ�����ͼ�Ľ�����һ���������������������������������ڷ�һ��ƶ�����Ľ����ƣ�������ú�ͣ���������β����һ�Ž���NaOH��Һ�������ȸ���Ԥ�ȣ��������ڳ�Բ��ʱ������ͨ�����������ɼ����Ż�ȼ�գ����ɴ������̣���������������ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
48 22 |
50 22 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com