
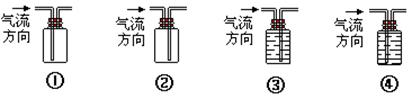
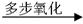 RCH2COOH����ͼ��ʾ�л���A��B��C��D��E��F��X��Y֮���ת���ϵ��
RCH2COOH����ͼ��ʾ�л���A��B��C��D��E��F��X��Y֮���ת���ϵ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

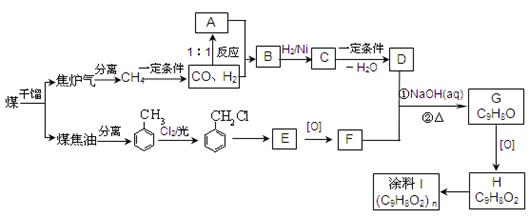
 H�ĺϳ�·�߲�ע����Ӧ������
H�ĺϳ�·�߲�ע����Ӧ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
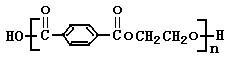
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
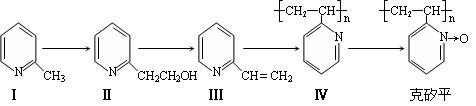
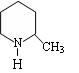
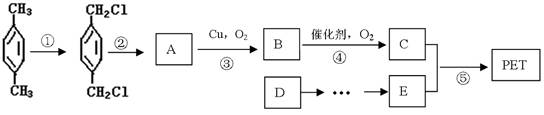
 ȷ���� ������ĸ��
ȷ���� ������ĸ��| A��������������CH3COOH����������Ӧ |
| B�������������������������� |
| C������������ʹ������Ȼ�̼��Һ��ɫ |
| D�����������ʹ���Ը��������Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����CH3��3C��CH2Cl | B��CH3Cl | C�� | D��CH2Cl��CH2Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
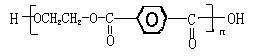 ������A��B���ֵ������۶��ɡ���֪�ױ����ڸ�����ش����������ɱ����ᡣ��Ҫ�Զ��ױ�����ϩΪԭ�ϣ����Լ�����ѡ�ϳɾ�����ά(��ȷ��)��
������A��B���ֵ������۶��ɡ���֪�ױ����ڸ�����ش����������ɱ����ᡣ��Ҫ�Զ��ױ�����ϩΪԭ�ϣ����Լ�����ѡ�ϳɾ�����ά(��ȷ��)���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com