
��8�֣���֪��
![]() I H2O2���н�ǿ�������ԣ�һЩ�ϲ����ý�����ϡ������ڵ�����£��ܺ�H2O2����������ԭ��Ӧ��
I H2O2���н�ǿ�������ԣ�һЩ�ϲ����ý�����ϡ������ڵ�����£��ܺ�H2O2����������ԭ��Ӧ��
II ����I2��������Һ����ɫ��
�������п�ͼ�ش����⣨����ʱ������ʽ�е�M��E������Ӧ��Ԫ�ط��ű�ʾ����

��д��M����ϡH2SO4��H2O2���Һ�Ļ�ѧ����ʽ�� �� ��
��ijͬѧȡX����Һ����������ϡ�����ữ��¶���ڿ�����һ��ʱ�䣬��ɫ��ƣ��ټ���KI��������Һ���ֱ�Ϊ��ɫ��д���������仯������ص����ӷ���ʽ�� �� �� �� ��
��д��Cl2��Z����ΪK2EO4�Ļ�ѧ����ʽ�� �� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���֪�����и������ʶ���1~18��Ԫ����ɣ�����֮��Ĺ�ϵ����ͼ��ʾ��
�����£�A��FΪ�������ʣ�J��Һ�壬F������L��Һ��Ӧ��������N��Һ��Ӧ��
C��H��MΪ���嵥�ʣ�����H�ʻ���ɫ��A��B��I��K��L��R����ɫ��Ӧ���ʻ�ɫ��
R��ˮ��Һ�еμ�����ʱ���տ�ʼ�а�ɫ�������������������ܽ⡣
��֪G��H2O2��H2O2�ڼ��������·ֽ����ʻ�ӿ졣��ش�
��1��BΪ����ɫ���壬��д��B��J��Ӧ�����ӷ���ʽ
��2��P���ȶ��ֽ��N��C���÷�Ӧ�Ļ�ѧ����ʽΪ
��3����д��������ⷴӦ�Ļ�ѧ����ʽ
��4��R���������ᷴӦ�����ӷ���ʽΪ
��5��F�dz�����Ұ�⺸���������ԭ�ϣ���д�����������������Ļ�ѧ��Ӧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��10�֣���֪�����и������ʶ���1~18��Ԫ����ɣ�����֮��Ĺ�ϵ����ͼ��ʾ��
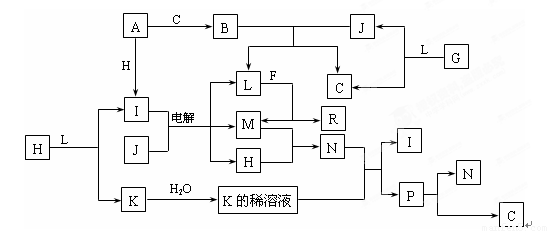
�����£�A��FΪ�������ʣ�J��Һ�壬F������L��Һ��Ӧ��������N��Һ��Ӧ��
C��H��MΪ���嵥�ʣ�����H�ʻ���ɫ��A��B��I��K��L��R����ɫ��Ӧ���ʻ�ɫ��
R��ˮ��Һ�еμ�����ʱ���տ�ʼ�а�ɫ�������������������ܽ⡣
��֪G��H2O2��H2O2�ڼ��������·ֽ����ʻ�ӿ졣��ش�
��1��BΪ����ɫ���壬��д��B��J��Ӧ�����ӷ���ʽ
��2��P���ȶ��ֽ��N��C���÷�Ӧ�Ļ�ѧ����ʽΪ
��3����д��������ⷴӦ�Ļ�ѧ����ʽ
��4��R���������ᷴӦ�����ӷ���ʽΪ
��5��F�dz�����Ұ�⺸���������ԭ�ϣ���д�����������������Ļ�ѧ��Ӧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�ڽμ���Ի�ѧ�Ծ� ���ͣ������
��֪�����и������ʶ���1~18��Ԫ����ɣ�����֮��Ĺ�ϵ����ͼ��ʾ��

�����£�A��FΪ�������ʣ�J��Һ�壬F������L��Һ��Ӧ��������N��Һ��Ӧ��C��H��MΪ���嵥�ʣ�����H�ʻ���ɫ��A��B��I��K��L��R����ɫ��Ӧ���ʻ�ɫ��R��ˮ��Һ�еμ�����ʱ���տ�ʼ�а�ɫ�������������������ܽ⡣��֪G��H2O2��H2O2�ڼ��������·ֽ����ʻ�ӿ졣��ش�
��1��BΪ����ɫ���壬��д��A��C��Ӧ����B�Ļ�ѧ����ʽ
��2��P���ȶ��ֽ��N��C���÷�Ӧ�Ļ�ѧ����ʽΪ
��3����д��������ⷴӦ�Ļ�ѧ����ʽ
��4��R���������ᷴӦ�����ӷ���ʽΪ
��5��ij����Q����Ư�����ã���Q��H�����ʵ���ͨ��ˮ��������Һû��Ư�����ã�д�����������ӷ�Ӧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣���֪��
I H2O2���н�ǿ�������ԣ�һЩ�ϲ����ý�����ϡ������ڵ�����£��ܺ�H2O2����������ԭ��Ӧ��
II ����I2��������Һ����ɫ��
�������п�ͼ�ش����⣨����ʱ������ʽ�е�M��E������Ӧ��Ԫ�ط��ű�ʾ����

��д��M����ϡH2SO4��H2O2���Һ�Ļ�ѧ����ʽ�� ��
��ijͬѧȡX����Һ����������ϡ�����ữ��¶���ڿ�����һ��ʱ�䣬��ɫ��ƣ��ټ���KI��������Һ���ֱ�Ϊ��ɫ��д���������仯������ص����ӷ���ʽ�� �� ��
��д��Cl2��Z����ΪK2EO4�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com