
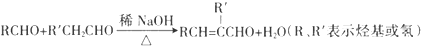
 ��
��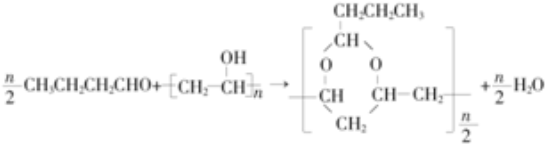 ��
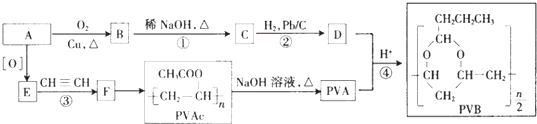
������ AΪ����һԪ������ͨʽΪCnH2n+2O����������������ԼΪ34.8%������$\frac{16}{12n+2n+2+16}$��100%=34.8%������n=2������AΪCH3CH2OH���������и�����ת����ϵ��A������EΪCH3COOH��E����Ȳ�����ӳɷ�Ӧ��FΪCH3COOCH=CH2��F�����Ӿ۷�Ӧ��PVAc������ˮ���PVAΪ ��A��ͭ��������������������BΪCH3CHO��B������Ϣ���еķ�Ӧ��CΪCH3CH=CHCHO��C������ԭ��Ӧ��DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB���ݴ˴��⣮
��A��ͭ��������������������BΪCH3CHO��B������Ϣ���еķ�Ӧ��CΪCH3CH=CHCHO��C������ԭ��Ӧ��DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB���ݴ˴��⣮
��� �⣺AΪ����һԪ������ͨʽΪCnH2n+2O����������������ԼΪ34.8%������$\frac{16}{12n+2n+2+16}$��100%=34.8%������n=2������AΪCH3CH2OH���������и�����ת����ϵ��A������EΪCH3COOH��E����Ȳ�����ӳɷ�Ӧ��FΪCH3COOCH=CH2��F�����Ӿ۷�Ӧ��PVAc������ˮ���PVAΪ ��A��ͭ��������������������BΪCH3CHO��B������Ϣ���еķ�Ӧ��CΪCH3CH=CHCHO��C������ԭ��Ӧ��DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB��
��A��ͭ��������������������BΪCH3CHO��B������Ϣ���еķ�Ӧ��CΪCH3CH=CHCHO��C������ԭ��Ӧ��DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB��
��1����������ķ�����֪��A�Ļ�ѧ����Ϊ�Ҵ���PVA�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ���Ҵ��� �� ��������
�� ��������
��2��CΪCH3CH=CHCHO��C�й����ŵ�������̼̼˫����ȩ����A��F�к˴Ź������׳���������C��D������4�ַ壬
�ʴ�Ϊ��̼̼˫����ȩ����C��D��
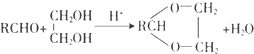
��3����Ӧ�۵Ļ�ѧ����ʽΪCH3COOH+CH��CH��CH3COOCH=CH2����Ӧ�����Ǽӳɷ�Ӧ����Ӧ�ܵĻ�ѧ����ʽΪ  ��
��
�ʴ�Ϊ��CH3COOH+CH��CH��CH3COOCH=CH2���ӳɷ�Ӧ�� ��
��
��4��FΪCH3COOCH=CH2����F������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪ��HCOOCH=CHCH3��HCOOCH2CH=CH2��CH3OOCCH=CH2��HCOOC��CH3��=CH2��
�ʴ�Ϊ��HCOOCH=CHCH3��HCOOCH2CH=CH2��CH3OOCCH=CH2��HCOOC��CH3��=CH2���������֣���
���� ���⿼���л���ĺϳɣ�Ϊ�߿��������ͣ��ۺϿ���ѧ�������������ۺ����û�ѧ֪ʶ����������Ŀ�ѶȽϴ����ʱע��������е���Ϣ��ע������л�������ŵĽṹ�����ʣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ǽ�����Cl��Br���ʽ�Cl2ͨ��NaBr��Һ�У�������ӦΪ��Cl2+2Br-=Br2+2Cl- | |
| B�� | �ǽ�����F��Br�������ԣ�HF��HBr | |
| C�� | �ǽ�����S��33As����ǰ�ߵ���̬�⻯���ȶ��Ը�ǿ | |
| D�� | �ǽ�����O��N����O2��H2���ϱ�N2������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������Ư�۾�����Ҫ�ɷ� | |
| B�� | �����ƿ����ں������ | |
| C�� | ���������Ӽ���ʳ������彡�����к�������ʳ�� | |
| D�� | ��������������Դ����������β����������������߿������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
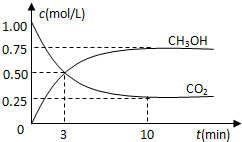 �����Ϊ1L���ܱ������У�������䣩����1mol CO2��3mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g�� ���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ������˵����ȷ���ǣ�������
�����Ϊ1L���ܱ������У�������䣩����1mol CO2��3mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g�� ���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ���е�3����ʱ������Ӧ���ʺ��淴Ӧ������� | |
| B�� | 10���Ӻ������и�����Ũ�Ȳ��ٸı� | |
| C�� | �ﵽƽ��������¶ȣ�����Ӧ���������淴Ӧ���ʼ�С | |
| D�� | 3minǰv����v����3min��v����v�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | Na2S04��Һ | AgN03��Һ | ���� | ||
| ���/mL | Ũ��/��mol•L-1�� | ���/�� | Ũ��/��mol•L-1�� | ||
| �� | 1 | l | 3 | 2 | ���ִ�����ɫ���� |
| �� | 1 | 1 | 3 | 0.5 | ����������ɫ���� |
| �� | 1 | 1 | 3 | 0.1 | �������� |
| �� | 1 | 1 | 3 | 0.0l | �����Ա仯 |
| ��� | AgNO3Ũ��/��mol•L-1�� | ϡ�ͺ�Ag+Ũ��/��mol•L-1�� | ���Һ��SO42-����С���ۼ��Ũ��/��mol•L-1�� |
| �� | 2 | 0.2 | 0.0003 |
| �� | 0.5 | 0.0048 | |
| �� | 0.1 | 0.0l | 0.12 |
| �� | 0.001 |
| ��� | AgNO3��Һ Ũ��/��mol•L-1�� | ���� | ������еμ����������� |
| �� | 2 | ���ִ�����ɫ���� | �μ�ϡ���ᣬ���������ܽ⣻�ļ�Ũ���ᣬ�����Ͽ���ʧ |
| �� | 0.5 | ����������ɫ���� | �μ�ϡ���ᣬ����������ʧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HCl��ǿ���H2SO3������ | B�� | HClO4�����Ա�H2SO4ǿ | ||
| C�� | H2S��HCl�ȶ� | D�� | H2SO4��HClO����ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| �� | �� | �� | �� | |||||
| �� | �� | �� | �� | �� | �� | �� | ||
| �� | �� |
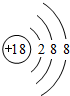 ��
��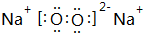 �����к��еĻ�ѧ�������Ӽ������ۼ���
�����к��еĻ�ѧ�������Ӽ������ۼ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
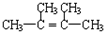 ��
��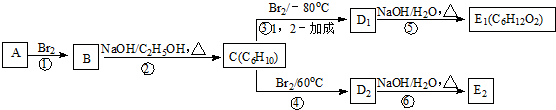
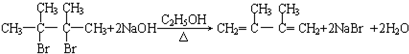 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com