
����Ŀ����Ԫ�ص��⻯����������ڹ�ҵ�������������ж��й㷺Ӧ�ã��ش��������⣺
(1)��Ԫ��ԭ�ӵ� L �������Ϊ ___________��
(2)��(N2H4)�ֳ���������ɫ��״Һ�塣�������ڰ��Ĵ̱���ζ������Ϊ�����������ȼ�ϡ�
��)���е�Ԫ�صĻ��ϼ�Ϊ ____��
��NH3 �� NaClO ��Ӧ�ɵõ��£��÷�Ӧ�Ļ�ѧ����ʽΪ_____��
��16g Һ̬���ڿ�����ȼ�գ����ɵ�����ˮ����ʱ�ų�������Ϊ 267.1kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ�� ____��
���𰸡�5 -2 2NH3+NaClO === N2H4 + NaCl + H2O N2H4(1)+ O2(g)=== N2(g)+ 2H2O(g),��H=-534.2kJmol-1
��������
(1)����ԭ�Ӻ�����ӷֲ��Ų��Ĺ��ɿ�ȷ����ԭ�ӵ�L���������
(2) ��������Ԫ����-1�ۣ����ݻ������и�Ԫ�ػ��ϼ۴�����Ϊ0��ȷ����Ԫ�صĻ��ϼۣ��ڸ���NaClO����ǿ��������NH3�е�Ԫ����-3�ۣ��л�ԭ�ԣ�����֪NaClO�ܽ�NH3���������ݵ�ʧ�����غ��ԭ���غ���ƽ������ȷ��1molN2H4��ȫȼ�շų�����������д���Ȼ�ѧ����ʽ��
(1)��Ԫ�ص�ԭ�Ӻ�����7�����ӣ������������ԭ����K����2�����ӣ�L����5��������
(2) ��������Ԫ����-1�ۣ����µķ���ʽN2H4��֪����Ԫ�صĻ��ϼ�Ϊ-2�ۣ�����NaClO���н�ǿ�������ԣ���Ӧ��ͨ����Ԫ����+1�۱仯��-1�ۣ��õ����ӣ�NH3ת��ΪN2H4�Ĺ����е�Ԫ�صĻ��ϼ���-3�۱仯��-2����ʧȥ���������Ը÷�ӦΪ������ԭ��Ӧ��1��NaClOת��Ϊ1��NaCl�õ�2�����ӣ�2��NH3����1���·���ʧȥ2�����ӣ����ݵ��ӵ�ʧ�غ��ԭ���غ���ƽ���仯ѧ��Ӧ����ʽΪ��2NH3+NaClO = N2H4 + NaCl + H2O�����µ�Ħ������=32g/mol��16gN2H4�����ʵ���=![]() =0.5mol��1molN2H4��ȫȼ�շų�������=267.1kJ��
=0.5mol��1molN2H4��ȫȼ�շų�������=267.1kJ��![]() =534.2kJ�����Ȼ�ѧ����ʽΪ��N2H4(l)+O2(g)=N2(g)+2H2O(g)����H=-534.2kJmol-1��
=534.2kJ�����Ȼ�ѧ����ʽΪ��N2H4(l)+O2(g)=N2(g)+2H2O(g)����H=-534.2kJmol-1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ȷ�Ӧ��ʵ��װ����ͼ�������й����ȷ�Ӧ��˵���У�����ȷ����
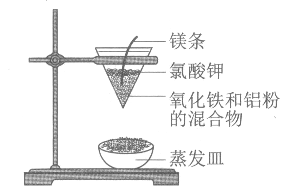
A. ���ȷ�Ӧ�Ƿ��ȷ�Ӧ
B. ���ȷ�Ӧ������ұ��ijЩ���۵����
C. ʵ����þ������Ҫ�����ǻ�ԭ������
D. ʵ������Ϊ�����Ľ���©���·��к�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶��£����淴ӦA(��)+3B(��)![]() 2C(��)���ﵽƽ��ı�־��(����)
2C(��)���ﵽƽ��ı�־��(����)
A. C������������B�������������B. ��������ƽ����Է�����������
C. ��λʱ��������nmolA��ͬʱ����3nmolBD. A��B��C�ķ�����֮��Ϊ1:3:2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A.������������ˮ������B.������̼ͨ��Na2SiO3��Һ�п��Եõ�����
C.�������賣���������D.����������������������������κ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ȼ��⣨ICl���Ļ�ѧ���ʸ��������ƣ�Ԥ������ˮ��Ӧ�������������
A.HI��HClOB.HCl��HIOC.HClO3��HIOD.HClO��HIO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ȼ���(ICl3)��ҩ��ϳ�����;�dz��㷺�����۵㣺33 �����е㣺73 ����ʵ���ҿ�����ͼװ����ȡ ICl3��
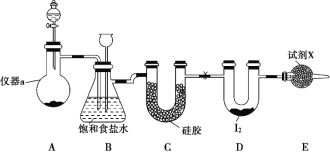
(1)���� a ��������_____��
(2)�Ʊ�����ѡ�õ�ҩƷΪƯ������[ ��Ҫ�ɷ�Ϊ Ca(ClO)2] ��Ũ���ᣬ ��ط�Ӧ�Ļ�ѧ����ʽΪ______��
(3)װ�� B �����ڳ��ӣ�Ҳ�ǰ�ȫƿ���ܼ��ʵ�����ʱװ�� C ���Ƿ�����������д����������ʱ B �е������� ____��
(4)�Լ� X Ϊ_____��
(5)�����뵥�ʵⷴӦ�¶��Ե��� 70 ������װ�� D ���˵ļ��ȷ�ʽΪ__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й������ʷ������ȷ����� ( )
�� | �� | �� | ���������� | ���������� | |
A | Na2CO3 | H2SO4 | (NH4)2CO3 | MgO | CO2 |
B | NaOH | HCl | NaCl | Na2O | H2O |
C | Ba(OH)2 | H2CO3 | CaCl2 | CO2 | SO2 |
D | KOH | HNO3 | CaCO3 | CaO | SO3 |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������ڵ���ʵ��� ( )
A. �ƾ� B. ϡ���� C. �Ȼ��� D. ����������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ǿ�����ڡ�2018�����Ժ�����������桷��ǿ����������������������ŷ���Ҫ�½�3%������ˣ��о�����������(��NOx)������(��SO2)�������Ż����Ļ������塣
��1���������������ϰ�װ����ת���������䷴Ӧ���Ȼ�ѧ����ʽΪ��2NO(g)+2CO(g)![]() 2CO2(g)+N2(g) ��H=-746.50kJ��mol-1��T��ʱ���������ʵ�����NO��CO�����ݻ�Ϊ2L���ܱ������У����¶Ⱥ�������䣬��Ӧ������(0~15min) NO�����ʵ�����ʱ��仯��ͼ��
2CO2(g)+N2(g) ��H=-746.50kJ��mol-1��T��ʱ���������ʵ�����NO��CO�����ݻ�Ϊ2L���ܱ������У����¶Ⱥ�������䣬��Ӧ������(0~15min) NO�����ʵ�����ʱ��仯��ͼ��

��ͼ��a��b�ֱ��ʾ����ͬ�¶��£�ʹ��������ͬ���������ͬ�Ĵ���ʱ���ﵽƽ�������n (NO)�ı仯���ߣ����б�ʾ����������ϴ��������___________�����a����b����
��T��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=_______________��ƽ��ʱ�������¶Ȳ��䣬���������г���CO��CO2��0.2 mol����ƽ�⽫_________�ƶ���(����������ҡ�����)
��15minʱ�����ı���練Ӧ����������n (NO)����ͼ����ʾ�仯����ı������������_______________________________________________ (�δ�һ������)��
��2���ڴ��������£��û�ԭ��[����(N2H4)]ѡ���Ե���NOx��Ӧ����N2��H2O��
��֪200��ʱ����.3N2H4(g)=N2(g)+4NH3(g) ��H1=-32.9 kJ��mol-1��
II. N2H4(g)+H2(g) =2NH3(g) ��H2=-41.8 kJ��mol-1��
��д���µĵ���ʽ��____________________��
��200��ʱ���·ֽ�ɵ������������Ȼ�ѧ����ʽΪ��_____________________________��
��Ŀǰ����ѧ�������о�һ������ϩ��Ϊ��ԭ��������ԭ���������������¶ȡ�������(����ɸ�д�������������)�Ĺ�ϵ����ͼ��ʾ��

Ϊ�ﵽ�������Ч����Ӧ��ȡ��������_________________________________________��
��3�����õ��װ��Ҳ�ɽ���������������ͼ�ɽ������е�NO��SO2�ֱ�ת��ΪNH4+��SO42-�������ĵ缫��ӦʽΪ____________________________������A��______________ (�ѧʽ)��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com