
�������ӷ���ʽ��д��ȷ����(����)
A��Cu(OH)2������OH����H��===H2O
B���Ƽ���ˮ�У�Na��2H2O===Na����2OH����H2��
C��FeSO4��Һ�м���ϡ���3Fe2����4H����NO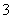 ===3Fe3����2H2O��NO��
===3Fe3����2H2O��NO��
D��Al2(SO4)3��Һ�м�������Ba(OH)2��Һ��2Al3����3SO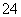 ��3Ba2����6OH��===2Al(OH)3����3BaSO4��
��3Ba2����6OH��===2Al(OH)3����3BaSO4��
 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijϡ�����ϡ����Ļ����Һ200 mL��ƽ���ֳ����ݡ�������һ��������ͭ�ۣ�������ܽ�19.2 g(��֪����ֻ����ԭΪNO����)������һ�����������ۣ�������������������������ӵı仯������ͼ��ʾ�����з����������(����)
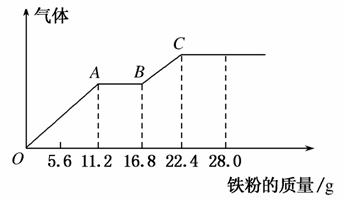
A��AB�εķ�ӦΪ��Fe��2Fe3��===3Fe2��
B���ڶ�����Һ����������ΪFeSO4
C���������NO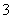 ���ʵ���Ϊ0.4 mol
���ʵ���Ϊ0.4 mol
D���������H2SO4Ũ��Ϊ5 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ��mA(g)+nB(g) pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����:
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����:
(1)�÷�Ӧ���淴ӦΪ_________�ȷ�Ӧ��m+n_________p(�������=��������)��
(2)��ѹʱ��A����������_________��(�������С�����䡱����ͬ)
(3)������B(�������)����A��ת����_________��B�ĵ�ת����_________��
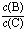 (4)�������¶ȣ���ƽ��ʱB��C��Ũ��֮�� ��______ ___��
(4)�������¶ȣ���ƽ��ʱB��C��Ũ��֮�� ��______ ___��
(5)�����������ƽ��ʱ��������������ʵ���____ _____��
(6)��B����ɫ���ʣ�A��C����ɫ�������C(�������)ʱ�������ɫ______ _����ά��������ѹǿ���䣬��������ʱ���������ɫ____ ___(��������dz�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Һ��XO42- ��SO32- ��������֮��Ϊ1��2ʱ��ǡ����ȫ����������ԭ��Ӧ��X�ڻ�ԭ�����еĻ��ϼ�Ϊ �� ��
A�� +1 B��+2 C��+3 D��+4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ��ȤС���ڿ����У���ij��Һ�����˶�μ�⣬�������μ�������±���ʾ��
| ������ | ��Һ�м��������� |
| ��1�� | KCl��K2SO4��Na2CO3��NaCl |
| ��2�� | KCl��BaCl2��Na2SO4��K2CO3 |
| ��3�� | Na2SO4��KCl��K2CO3��NaCl |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ����������һ�ֽ�����������(��������Դ��ˮ���������)����Һ������Һ���������ӵ�Ũ�Ⱦ���ȣ��ܴﵽ��Ŀ�ĵ���(����)
A��Na����Mg2����SO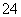 ��Cl��
��Cl��
B��ClO����I����NH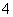 ��Ba2��
��Ba2��
C��Na����AlO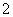 ��K����HCO
��K����HCO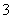
D��Al3����K����SO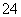 ��NO
��NO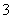
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȡm gþ���Ͻ���һ��Ũ�ȵ�ϡ������ǡ����ȫ�ܽ�(����Ļ�ԭ����ֻ��NO)����Ӧ��Ļ����Һ�еμ�b mol/L NaOH��Һ�����μӵ�V mLʱ���õ���������ǡ��Ϊ���ֵn g���������йظ�ʵ���˵������ȷ����(����)
�ٳ�����OH��������Ϊ(n��m)g
��ǡ���ܽ����Һ�е�NO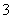 �����ʵ���Ϊ
�����ʵ���Ϊ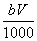 mol
mol
�۷�Ӧ������ת�Ƶĵ���Ϊ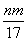 mol
mol
�ܱ�״��������NO�����Ϊ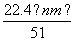 L
L
����Ͻ�Ӧ����������ʵ���Ϊ mol
mol
A��5�����B��4�����C��3�����D��2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£�0.01 mol��L��1 MOH��Һ��pHΪ10����֪��2MOH(aq)��H2SO4(aq)===M2SO4(aq)��2H2O(l)����H1����2 4.2 kJ��mol��1��H��(aq)��OH��(aq)===H2O(l)����H2����57.3 kJ��mol��1����MOH��ˮ��Һ�е���Ħ�HΪ(����)
4.2 kJ��mol��1��H��(aq)��OH��(aq)===H2O(l)����H2����57.3 kJ��mol��1����MOH��ˮ��Һ�е���Ħ�HΪ(����)
A����33.1 kJ��mol��1 B����45.2 kJ��mol��1
C����81.5 kJ��mol��1 D����33.1 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ������ȷ����(����)
A������������������Fe(OH)3��3H��===Fe3����3H2O
B��С�մ���Һ�ʼ��Ե�ԭ��HCO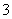 ��H2OH3O����CO
��H2OH3O����CO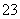
C���廯������Һ��ͨ������������2Fe2����4Br����3Cl2===2Fe3����2Br2��6Cl��
D�������������Һ�еμ�����Ba(OH)2��Һ��NH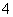 ��Al3����2SO
��Al3����2SO ��2Ba2����5OH��===AlO
��2Ba2����5OH��===AlO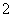 ��2BaSO4����NH3·H2O��2H2O
��2BaSO4����NH3·H2O��2H2O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com