

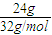 =0.75mol��������Ӧ�Ļ�ѧ����ʽΪ��2H2O2
=0.75mol��������Ӧ�Ļ�ѧ����ʽΪ��2H2O2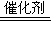 2H2O+O2��������1molO2ת�Ƶĵ�����2mol��0.75mol��������ת�Ƶ���Ϊ=0.75mol×2×6.02×1023=9.03x1023��
2H2O+O2��������1molO2ת�Ƶĵ�����2mol��0.75mol��������ת�Ƶ���Ϊ=0.75mol×2×6.02×1023=9.03x1023��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��b+c![]() Y
Y
��a+c![]() Z
Z
��X+Y![]() m
m
��X+Z![]() c+n
c+n
��Y+Z![]() c+n
c+n
��1��������Ӧ�У�����������˵���÷�Ӧһ������������ԭ��Ӧ����____________���Ӧ��ţ���
��2�����m��һ����ɫ����״Һ�廯�����X��Y��Z�Ļ�ѧʽ�ֱ���____________��____________��____________��
��3���۷�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
��4���ܷ�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.B+C![]() Y�� ��.A+C
Y�� ��.A+C![]() Z�� ��.X+Y
Z�� ��.X+Y![]() M�� ��.X+Z
M�� ��.X+Z![]() C+N; ��.Y+Z
C+N; ��.Y+Z![]() C+N
C+N
��1��������Ӧ�У�һ������������ԭ��Ӧ����______________�����Ӧ��ţ�
��2�����������M��һ����ɫ����״Һ�壬��X��Y�Ļ�ѧʽ�ֱ���______________��______________��
��3��Z�ĵ���ʽΪ______________��
��4��д����Ӧ��Ļ�ѧ����ʽ��__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����������ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
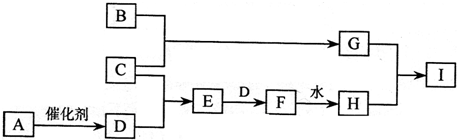
��a��b��c���ֳ����Ķ�����Ԫ�أ�����֮��������Ϲ��ɻ�����X��Y��Z��X��Y��Z֮��Ҳ���������Ӧ����֪X����a��bԪ�ذ�ԭ�Ӹ�����1��1��ɵĻ����a��b��cԪ���γɵĵ��ʣ�����a��b��c��ʾ������������ɵĻ�����֮��ķ�Ӧ��ϵ���£�δ��ƽ������b+c Y ��a+c
Y ��a+c Z ��X+Y
Z ��X+Y m ��X+Z
m ��X+Z c+n ��Y+Z
c+n ��Y+Z c+n
c+n
(1)������Ӧ�У�����������˵���÷�Ӧһ������������ԭ��Ӧ����____(����)��
(2)���m��һ����ɫ����״Һ�廯�����X�Ļ�ѧʽ��____________��
(3)�۷�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
(4) �ݷ�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com