
ėĀ(N2H4)ÓÖ³ĘĮŖ°±£¬ŹĒŅ»ÖÖæÉČ¼ŠŌŅŗĢ壬ÓėŃõĘų»ņµŖŃõ»ÆĪļ·“Ó¦¾łæÉÉś³ÉµŖĘųŗĶĖ®£®ĒāĘųŹĒŅ»ÖÖĒå½ąÄÜŌ“£¬ŅŗĒāŗĶėĀ¾łæÉÓĆ×÷»š¼żČ¼ĮĻ£®
¢ńĒāĘųµÄÖĘČ”Óė“¢“ęŹĒĒāÄÜŌ“ĄūÓĆĮģÓņµÄŃŠ¾æČČµć£®
ŅŃÖŖ£ŗCH4(g)£«H2O(g)£½CO(g)£«3H2(g)””¦¤H£½£«206.2 kJ”¤mol£1
CH4(g)£«CO2(g)£½2CO(g)£«2H2(g)¦¤H£½£«247.4 kJ”¤mol£1
(1)ĒāĘų×÷ĪŖŠĀÄÜŌ“µÄÓŵć________£®(“š2µć)
(2)ŅŌ¼×ĶéĪŖŌĮĻÖĘČ”ĒāĘųŹĒ¹¤ŅµÉĻ³£ÓƵÄÖĘĒā·½·Ø£®CH4(g)ÓėH2O(g)·“Ӧɜ³ÉCO2(g)ŗĶH2(g)µÄČČ»Æѧ·½³ĢŹ½ĪŖ________£®
(3)H2OµÄČČ·Ö½āŅ²æɵƵ½H2£¬øßĪĀĻĀĖ®·Ö½āĢåĻµÖŠÖ÷ŅŖĘųĢåµÄĢå»ż·ÖŹżÓėĪĀ¶ČµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®Ķ¼ÖŠA”¢B±ķŹ¾µÄĪļÖŹŅĄ“ĪŹĒ________”¢________£®

¢ņ(4)ėĀŅ»æÕĘųČ¼ĮĻµē³ŲŹĒŅ»ÖÖ¼īŠŌČ¼ĮĻµē³Ų£¬µē½āÖŹČÜŅŗŹĒ20£„£30£„µÄKOHČÜŅŗ£®øƵē³Ų·ÅµēŹ±£¬øŗ¼«µÄµē¼«·“Ó¦Ź½ŹĒ________£®
(5)ĻĀĶ¼ŹĒŅ»øöµē»ÆѧװÖĆŹ¾ŅāĶ¼£®ÓĆėĀŅ»æÕĘųČ¼ĮĻµē³Ų×ö“Ė×°ÖƵĵēŌ“£®

¢ŁČē¹ūAŹĒ²¬µē¼«£¬BŹĒŹÆÄ«µē¼«£¬CŹĒĮņĖį£ĮņĖįļ§£¬Ņõ¼«µÄµē¼«·“Ó¦Ź½ŹĒ________£®
¢ŚĄūÓĆøĆ×°ÖĆæÉÖʵĆÉŁĮæ¹żŃõ»ÆĒā£ŗŌŚŃō¼«ÉĻSO42£±»Ńõ»Æ³ÉS2O82£(¹ż¶žĮņĖįøłĄė×Ó)£¬S2O82£ÓėH2O·“Ӧɜ³ÉH2O2£¬S2O82££«2H2O£½2SO42££«H2O2£«2H+£®ČōŅŖÖĘČ”2 molH2O2£¬øĆČ¼ĮĻµē³ŲĄķĀŪÉĻŠčĻūŗÄ________molN2H4£®
(6)ÓÉA”¢B”¢C”¢DĖÄÖÖ½šŹō°“ĻĀ±ķ֊װÖĆ½ųŠŠŹµŃ飮
ŹµŃé×°ÖĆÓėĻÖĻó

øł¾ŻŹµŃéĻÖĻó»Ų“šĻĀĮŠĪŹĢā£ŗ
¢Ł×°ÖƱūÖŠČÜŅŗµÄPH________£®(Ģī”°±ä“ó”±”°±äŠ””±»ņ”°²»±ä”±)
¢ŚĖÄÖÖ½šŹō»īĘĆŠŌÓÉČõµ½ĒæµÄĖ³ŠņŹĒ________£®

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)ėĀ(N2H4)ÓÖ³ĘĮŖ°±£¬ŹĒŅ»ÖÖæÉČ¼ŠŌµÄŅŗĢ壬æÉÓĆ×÷»š¼żČ¼ĮĻ”£ŅŃÖŖŌŚ101 kPaŹ±£¬32.0 g N2H4ŌŚŃõĘųÖŠĶźČ«Č¼ÉÕÉś³ÉµŖĘų£¬·Å³öČČĮæ624 kJ(25”ꏱ)£¬N2H4ĶźČ«Č¼ÉÕ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ŹĒ____________________________________________________________________”£
(2)ėĀ-æÕĘųČ¼ĮĻµē³ŲŹĒŅ»ÖÖ¼īŠŌČ¼ĮĻµē³Ų£¬µē½āÖŹŹĒ20%”Ŗ30%µÄKOHČÜŅŗ”£Š“³öėĀ-æÕĘųČ¼ĮĻµē³Ų·ÅµēŹ±Õż”¢øŗ¼«µÄµē¼«·“Ó¦Ź½”£
Õż¼«£ŗ________________________________£¬
øŗ¼«£ŗ________________________________
(3)Ķ¼2-2-5ŹĒŅ»øöµē»Æѧ¹ż³ĢŹ¾ŅāĶ¼”£
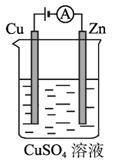
Ķ¼2-2-5
¢ŁŠæʬÉĻ·¢ÉśµÄµē¼«·“Ó¦ŹĒ________________________________________________”£
¢Ś¼ŁÉčŹ¹ÓĆėĀ?æÕĘųČ¼ĮĻµē³Ų×÷ĪŖ±¾¹ż³ĢÖŠµÄµēŌ“”¢ĶʬµÄÖŹĮæ±ä»Æ128 g£¬ŌņėĀ-æÕĘųČ¼ĮĻµē³ŲĄķĀŪÉĻĻūŗıź±ź×¼×“æöĻĀµÄæÕĘų___________L(¼ŁÉčæÕĘųÖŠŃõĘųĢå»żŗ¬ĮæĪŖ20%)
(4)“«Ķ³ÖʱøėĀµÄ·½·Ø£¬ŹĒŅŌNaClOŃõ»ÆNH3£¬ÖʵĆėĀµÄĻ”ČÜŅŗ”£øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗžÄĻŹ”ÖźÖŽŹŠøßČżµŚĖÄ“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ėĀ(N2H4)ÓÖ³ĘĮŖ°±£¬ŹĒŅ»ÖÖæÉČ¼ŠŌµÄŅŗĢ壬æÉÓĆ×÷»š¼żČ¼ĮĻ”£ŅŃÖŖ£ŗ
¢ŁN2(g) + 2O2(g) =2 NO2(g) ¦¤H = +67£®7kJ/mol
¢Ś2N2H4(g) + 2NO2(g) =3N2(g) + 4H2O (g) ¦¤H = £1135£®7kJ/mol
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A£®N2H4(g) + O2(g) = N2(g) + 2H2O(g) ¦¤H = £1068 kJ/mol
B£®ėĀŹĒÓė°±ĄąĖʵÄČõ¼ī£¬ĖüŅ×ČÜÓŚĖ®£¬ĘäµēĄė·½³ĢŹ½£ŗN2H4 + H2O = N2H5+ + OH-
C£®²¬×öµē¼«£¬ŅŌKOHČÜŅŗĪŖµē½āÖŹČÜŅŗµÄėĀ”Ŗ”ŖæÕĘųČ¼ĮĻµē³Ų£¬·ÅµēŹ±µÄøŗ¼«·“Ó¦Ź½£ŗN2H4 £4e£ + 4OH£ = N2 + 4H2O
D£®²¬×öµē¼«£¬ŅŌKOHČÜŅŗĪŖµē½āÖŹČÜŅŗµÄėĀ”Ŗ”ŖæÕĘųČ¼ĮĻµē³Ų£¬¹¤×÷Ņ»¶ĪŹ±¼äŗó£¬KOHČÜŅŗµÄpH½«Ōö“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗžÄĻŹ”ÖźÖŽŹŠøßČżµŚĖÄ“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ėĀ(N2H4)ÓÖ³ĘĮŖ°±£¬ŹĒŅ»ÖÖæÉČ¼ŠŌµÄŅŗĢ壬æÉÓĆ×÷»š¼żČ¼ĮĻ”£ŅŃÖŖ£ŗ
¢ŁN2(g) + 2O2(g) =2 NO2(g) ¦¤H = +67£®7kJ/mol
¢Ś2N2H4(g) + 2NO2(g) =3N2(g) + 4H2O (g) ¦¤H = £1135£®7kJ/mol
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A£®N2H4(g) + O2(g) = N2(g) + 2H2O(g) ¦¤H = £1068 kJ/mol
B£®ėĀŹĒÓė°±ĄąĖʵÄČõ¼ī£¬ĖüŅ×ČÜÓŚĖ®£¬ĘäµēĄė·½³ĢŹ½£ŗN2H4 + H2O = N2H5+ + OH-
C£®²¬×öµē¼«£¬ŅŌKOHČÜŅŗĪŖµē½āÖŹČÜŅŗµÄėĀ”Ŗ”ŖæÕĘųČ¼ĮĻµē³Ų£¬·ÅµēŹ±µÄøŗ¼«·“Ó¦Ź½£ŗN2H4 £4e£ + 4OH£ = N2 + 4H2O
D£®²¬×öµē¼«£¬ŅŌKOHČÜŅŗĪŖµē½āÖŹČÜŅŗµÄėĀ”Ŗ”ŖæÕĘųČ¼ĮĻµē³Ų£¬¹¤×÷Ņ»¶ĪŹ±¼äŗó£¬KOHČÜŅŗµÄpH½«Ōö“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗžÄĻŹ”ÖźÖŽŹŠøßČżµŚĖÄ“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ėĀ(N2H4)ÓÖ³ĘĮŖ°±£¬ŹĒŅ»ÖÖæÉČ¼ŠŌµÄŅŗĢ壬æÉÓĆ×÷»š¼żČ¼ĮĻ”£ŅŃÖŖ£ŗ
¢ŁN2(g) + 2O2(g) =2 NO2(g) ¦¤H = +67£®7kJ/mol
¢Ś2N2H4(g) + 2NO2(g) =3N2(g) + 4H2O (g) ¦¤H = £1135£®7kJ/mol
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A£®N2H4(g) + O2(g) = N2(g) + 2H2O(g) ¦¤H = £1068 kJ/mol
B£®ėĀŹĒÓė°±ĄąĖʵÄČõ¼ī£¬ĖüŅ×ČÜÓŚĖ®£¬ĘäµēĄė·½³ĢŹ½£ŗN2H4 + H2O = N2H5+ + OH-
C£®²¬×öµē¼«£¬ŅŌKOHČÜŅŗĪŖµē½āÖŹČÜŅŗµÄėĀ”Ŗ”ŖæÕĘųČ¼ĮĻµē³Ų£¬·ÅµēŹ±µÄøŗ¼«·“Ó¦Ź½£ŗN2H4 £4e£ + 4OH£ = N2 + 4H2O
D£®²¬×öµē¼«£¬ŅŌKOHČÜŅŗĪŖµē½āÖŹČÜŅŗµÄėĀ”Ŗ”ŖæÕĘųČ¼ĮĻµē³Ų£¬¹¤×÷Ņ»¶ĪŹ±¼äŗó£¬KOHČÜŅŗµÄpH½«Ōö“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğŗžÄĻŹ”ÖźÖŽŹŠøßČżµŚĖÄ“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ėĀ(N2H4)ÓÖ³ĘĮŖ°±£¬ŹĒŅ»ÖÖæÉČ¼ŠŌµÄŅŗĢ壬æÉÓĆ×÷»š¼żČ¼ĮĻ”£ŅŃÖŖ£ŗ
¢ŁN2(g) + 2O2(g) =2 NO2(g) ¦¤H = +67£®7kJ/mol
¢Ś2N2H4(g) + 2NO2(g) =3N2(g) + 4H2O (g) ¦¤H = £1135£®7kJ/mol
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A£®N2H4(g) + O2(g) = N2(g) + 2H2O(g) ¦¤H = £1068 kJ/mol
B£®ėĀŹĒÓė°±ĄąĖʵÄČõ¼ī£¬ĖüŅ×ČÜÓŚĖ®£¬ĘäµēĄė·½³ĢŹ½£ŗN2H4 + H2O = N2H5+ + OH-
C£®²¬×öµē¼«£¬ŅŌKOHČÜŅŗĪŖµē½āÖŹČÜŅŗµÄėĀ”Ŗ”ŖæÕĘųČ¼ĮĻµē³Ų£¬·ÅµēŹ±µÄøŗ¼«·“Ó¦Ź½£ŗN2H4 £4e£ + 4OH£ = N2 + 4H2O
D£®²¬×öµē¼«£¬ŅŌKOHČÜŅŗĪŖµē½āÖŹČÜŅŗµÄėĀ”Ŗ”ŖæÕĘųČ¼ĮĻµē³Ų£¬¹¤×÷Ņ»¶ĪŹ±¼äŗó£¬KOHČÜŅŗµÄpH½«Ōö“ó
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com