
���� ��1������m=cVM������Ҫ���ʵ�������
��2����������һ�����ʵ���Ũ����Һ��һ�㲽��ѡ����Ҫ������������������Һ���ѡ����Ҫ����ƿ���
��3������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ��ݴ�����
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����c=$\frac{n}{V}$������������
��5��n��Cl2��=0.1mol�����ݷ�Ӧ�����ӷ���ʽCl2+2OH-=CIO-+Cl?+H2O���㣻
��6����������һ�����ʵ���Ũ����Һ�IJ�����
��� �⣺��1������480mL0.2mol/L��NaOH��Һ����Ҫѡ��500mL����ƿ����Ҫ�������Ƶ�����m=0.2mol/L��0.5L��40g/mol=4.0g��
�ʴ�Ϊ��4.0��
��2������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ��õ���������������ƽ��ҩ�ס��ձ���������������ƿ����ͷ�ιܣ�����480mL0.2mol/L��NaOH��Һ��Ӧѡ��500mL����ƿ�����Ի�ȱ�ٵIJ���������500ml����ƿ��
�ʴ�Ϊ��500ml����ƿ��
��3������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ�������ȷ��˳��Ϊ��D H B F A E C G��
�ʴ�Ϊ��DHFAE��
��4���ٶ���ʱ������ˮ���������˿̶ȣ�������Һ���ƫ����ҺŨ��ƫ�ͣ�
������ƿ�������һ����ˮ�֣������ʵ����ʵ�������Һ������������Ӱ�죬��ҺŨ�Ȳ��䣻
�۶���ʱ���ӹ۲쵽Һ��պõ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ����ƫ�� ����Ӱ�� ��ƫ�ͣ�
��5��n��Cl2��=0.1mol���ɷ�Ӧ�����ӷ���ʽ��Cl2+2OH-=CIO-+Cl?+H2O��֪n��NaOH��=0.2mol������������Һ���V��NaOH��=$\frac{0.2mol}{0.2mol/L}$=1L=1000mL��
�ʴ�Ϊ��1000��
��6������100mL 1.0mol/L����IJ���Ϊ�����㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ�ѡ�õ��������Ⱥ�˳��Ϊ��10mL��Ͳ��50mL�ձ�����������100mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��CEFGH��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ���Ͳ��������ǽ���ؼ���ע���������ķ�������Ŀ�ѶȲ���


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �������� | HCl��aq�� | FeCl3 | NaOH | CH3COONa | C2H5OH |
| ��Һ��pH | 3 | 4 | 10 | 11 | δ�ⶨ |
| ˮ�ĵ���̶� | ��1 | ��2 | ��3 | ��4 | ��5 |
| A�� | ��3����1����5����4����2 | B�� | ��4����2����5����3����1 | C�� | ��2����4����5����1����3 | D�� | ��1����3����5����2����4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
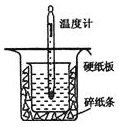 �к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�飮ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������50mL����ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺
�к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�飮ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������50mL����ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺| �¶� ʵ������� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | |
| 2 | 27.0 | 27.4 | 27.2 | 32.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO2���������ԣ�������Ư��ֽ�� | |
| B�� | �����������ԣ������ڵ�̲��� | |
| C�� | �������Ⱦ��л�ԭ�ԣ�����������ˮ��ɱ������ | |
| D�� | NH3���л�ԭ�ԣ�����NH3������CuO������ȡ����N2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
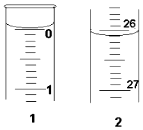 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�| �ζ� ���� | �������������� Һ�����/mL | 0.1000mol•L+1����������mL�� | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| ��һ�� | 25.00 | 0.00 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 |
| ������ | 25.00 | 0.22 | 26.31 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶�/�� | 200 | 300 | 400 |
| K | 1.0 | 0.85 | 0.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| Ԫ�ش��� | L | M | X | R | T | Q |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.102 | 0.089 | 0.074 | 0.078 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | +2 | -2 | +5��-3 |
| A�� | ���⻯��ķе㣺X��T��Q | |
| B�� | ���Ӱ뾶��X2-��T2-��L2+��M3+ | |
| C�� | ��ҵ���õ������״̬��L��T�Ļ�������ȡ����L | |
| D�� | L��M������������Ӧ��ˮ���������ǿ�Ӧ������ǿ�ᷴӦ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com