
��ȩ��ҽҩ�����������ϡ�ʳƷ������Ӧ�ù㷺���ϳɹ�ȩ���ù�ȩ�ϳɾ���F��·������ͼ��ʾ��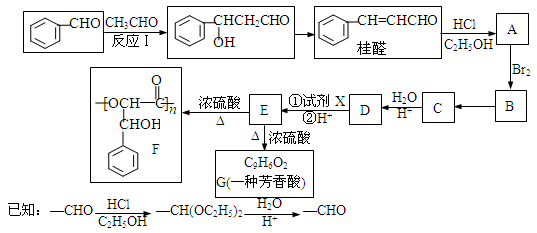
�����������������գ�
��1��д����Ӧ���͡���ӦB��C ����ӦE��G ��
��2��д���ṹ��ʽ��C ��G ��
��3�����Լ�XΪ����������ͭ����Һ ����÷�Ӧ�������� ����Ӧ������ _____________________ ��
��4����Ũ���ᡢ���ȵ�������E����F��ͬʱ��������G�Լ��������ɣ�д�����ֻ���һ�ֿ��ܵĽṹ��ʽ ��
��5����д��ͬʱ�������������Ĺ�ȩ������ͬ���칹��Ľṹ��ʽ ��
a�������в���ȩ�����ǻ� b�����ĶԶ�ȡ���� c���������⣬����������״�ṹ
��6��������Ӧ�õ��Ĺ�ȩ�к�����������ʽΪC11H12O2�Ļ�������������ɹ�ȩ����ȩ���������Ʒ�Ӧ��ķ�Ӧ���ɵġ�д����ȩ����ȩ����C11H12O2�Ļ�ѧ����ʽ ��
��1��ȡ����ˮ�⣩��(1��)������Ӧ
��2��
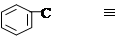 ��(1��) (1��)
��(1��) (1��)
��3�����ȷ��ڣ�����ש��ɫ�ij���(1��)
��4��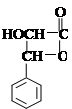 ��2�֣�
��2�֣�
��5��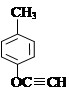
 ��2�֣���1�֣�
��2�֣���1�֣�
��6�� ��2�֣�
��2�֣�
�������������������Ϣ�ж�A��B��C��D��E�ֱ�Ϊ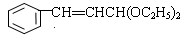 ��
��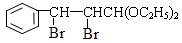 ��
��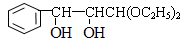 ��
��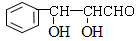 ��
��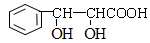 ��
��
��2���л���E��ȥ�����ǻ�����G��ֻ�����Ȼ�������Ӧ�γ�̼̼������
��4�������ﻷ�������Ƿ����ڳɻ���Ҳ���������ӳɻ����γ�������
��5�������в�����ȩ�����ǻ���ֻ�����ѣ���ȩ�к���2��˫�����ʸ�������ֻ�ܺ���̼̼������ֻ����2��̼ԭ����������
��6����Ӧ��Ӧ����Ϊ�ӳɷ�Ӧ����ȩ��H��CH2CHO�ӵ�ȩ����̼��˫���ϣ��ʸ÷�ӦΪ 
���㣺�����л��ϳ����������ơ�������жϡ�ͬ���칹����д���йط���ʽ��д���й����⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ɽ������һ�ֳ��õ�ʳƷ��������������ɽ�����һ�ֹ�ҵ�ϳ�;����
|
 ��֪����1��A�Ǻ���һ������ʯ�ͻ���ˮƽ����Ҫ����C�ķ���ʽΪC2H4O
��֪����1��A�Ǻ���һ������ʯ�ͻ���ˮƽ����Ҫ����C�ķ���ʽΪC2H4O


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
PTT�ǽ�������Ѹ�ٷ�չ���������������Ծ������ϣ������������ܣ�����Ϊ�������ϣ���֯��ά�͵�̺�Ȳ��϶��õ��㷺Ӧ�ã���ϳ�·�߿����Ϊ��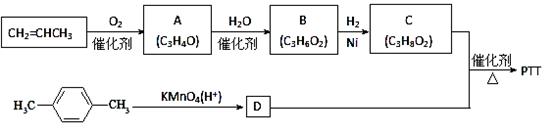
����A��B��C��Ϊ��״�����A ��B���ܷ���������Ӧ��C�в�������1mol C���������Ʒ�Ӧ����22��4 L H2 (��״��)��
��ش��������⣺
��1��A�����������ŵ�����Ϊ ��B�Ľṹ��ʽΪ ��
��2��������C��D��Ӧ����PTT�Ļ�ѧ����ʽΪ ����Ӧ����Ϊ ��
��3������ʽΪC4H6O����A��Ϊͬϵ���ͬ���칹���� �֣�
��4���벹������������CH2=CHCH3Ϊ��Ҫԭ��(���Լ�����)�Ʊ�CH3CH(OH)COOH�ĺϳ�·������ͼ(��ע����Ӧ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ѧ��R.F.Heck��������Heck��Ӧ�����2010��ŵ������ѧ����

�ش��������⣺
��1��C��ŨH2SO4��������F��F��ʹ����KMnO4��Һ��ɫ��F�Ľṹ��ʽ______��D��һ�������·�Ӧ��
�ɸ߷��ӻ�����G��G�Ľṹ��ʽ��___________________________��
��2����A��B�ķ�Ӧ�У�����A�Ƿ�Ӧ��ȫ���Լ���_______________________________��
��3��E��һ��ͬ���칹��K��������������������������ȡ�����ұ�����ֻ�����ֲ�ͬ��ѧ�������⣬��FeCl3��Һ��������ɫ��K�����NaOH��Һ���ȣ�������Ӧ�ķ���ʽΪ_________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����ѧʽΪC6H12��ijϩ��������̼ԭ�Ӷ���ͬһƽ���ϣ����ϩ���Ľṹ��ʽΪ ��������Ϊ ��ȡ�����������������ӳ����ò�����к˴Ź���������ں˴Ź�������ͼ��Ӧ�ó���_____ ���壬�������Ϊ ��һ���������
��2����״����1.68L��ɫ��ȼ������������������ȫȼ�ա���������ͨ����������ʯ��ˮ���õ���ɫ��������Ϊ15.0g������������ʯ������ȼ�ղ������9.3g����ԭ�����ǵ�һ���壬���ķ���ʽ ��
��3��ij�л����һ�ȴ��������������-CH3������-CH2-��һ�� ��һ��-Cl�������ܵĽṹ�� �֡������ⲻ���Ƕ�ӳ�칹�壩
��һ��-Cl�������ܵĽṹ�� �֡������ⲻ���Ƕ�ӳ�칹�壩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������������ȡ������ʧ��ҩ�����������м��塣
I����֪����������ṹ����ͼ ��ʾ��
��ʾ��
��1������������ĺ�������������Ϊ ��
��2�������й����������������������ȷ���� ������ĸ����
| A�����ܷ�����ȥ��Ӧ | B��������ˮ | C���뱽�ӻ�Ϊͬϵ�� | D��������ˮ��ӦE��1 mol����������������¼��������Ҫ4molH2F������̼������Һ��Ӧ����������̼ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������һ��ҩ��䳣�õĺϳ�·�����¡��ش��������⣺

��֪��
��1�������ӵĻ�ѧʽΪ ��
��2��A �� B��Ӧ������Լ��� �� B�� C��Ӧ�Ļ�ѧ����ʽΪ ��
��3��D��E��Ӧ���л���Ӧ������ ������E���й����ŵ������� ��
��4��E��F��Ӧ�Ļ�ѧ����ʽΪ ��
��5��д��ͬʱ��������������F��ͬ���칹��Ľṹ��ʽ�� ��
�ٺ��б��������ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ������ϡNaOH��Һ�У�1mol��ͬ���칹������2molNaOH������Ӧ���ܱ������ж��ֲ�ͬ��ѧ��������ԭ�ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������������л���A��B��C��D�������Ϣ��
����A�����е����к�̼�������������
����B��һ��ֲ���������ڼ��������ڴ����ʵ
����C��������ȼ�ղ�������Ȳ�泣�����и�ӽ���
��ҽ���ϳ����������Ϊ75%��D��Һ������
�ݴ˻ش��й����⣺
(1)��A��Cl2��ϳ���һ�Թܣ��ܷ�����ڹ�������һ��ʱ����ܿ����Թ��ڱ��ϳ��ֵ���״�ﲻ������__________(����)��
A.CH3Cl B.CH2Cl2 C.CHCl3 D.CCl4
��2��ʵ������ȡB�������¶ȹ��߶�ʹ�Ҵ���Ũ���ᷴӦ����������SO2��ijͬѧ�������ʵ����ȷ��������������к���B��SO2��
I.װ�ÿ�ʢ�ŵ��Լ��ǣ��ڣ� �ܣ� ���뽫�����й��Լ����������ո��ڣ�
A��Ʒ�� B��NaOH��Һ C��Ũ���� D������KMnO4��Һ
��.ȷ������B�������� ��
��3����C�ĽṹʽΪ ��
��4��D�м�������ᡢŨ���������H218O����һ��ʱ���18O������
�ٴ����ڱ������ˮ������ ��ֻ������ˮ������
�۴�����D�ͱ���������� �ܴ����ڱ������D������γɵ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������Ҫ�Ĺ�ҵԭ��֮һ����ҵ�ϲ��ñ��Ӻϳ��л���D��·�����£�
��ش��������⣺
(1)�л���A���ӹ������� (д����)��B�Ľṹ��ʽ�� ����˴Ź�������ͼ���� �ַ塣
(2)C��D�ķ�Ӧ������ ��D������NaOH��Һ����ʱ��Ӧ�Ļ�ѧ����ʽ��
(3)X��B��ͬ���칹�壬X�����к��б��������б�����һ�ȴ���ֻ�����֣����ܷ���������Ӧ����X�����нṹ��ʽ�� �� �� �� ��
�� �� �� ��
(4)�йػ�����C��˵����ȷ���� (����ĸ)
| A���ܷ����ӳɷ�Ӧ |
| B��һ�������£�������NaOH����Һ�з�����ȥ��Ӧ |
| C��1molC������NaOH��Һ���ȣ��������2molNaOH |
| D�������������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com