
������ͼ��ʾ��װ����ȡ�϶����ı�����ˮ��

�ش��й����⣺
(1)д���йط�Ӧ�����ӷ���ʽ��
װ�üף� ��װ�ö��� ��
(2)֤����ˮ�ѱ��͵������� ��
(3)��ȡ����ʱ��װ�ñ���Һ���к����������� (ˮ���ӳ���)��װ���ҵ������� ��
(4)�����¸Ľ���ʩ���飬���������ۣ�

����װ���Һͱ�֮��������ͼ(a)��ʾ��װ�ã�����Ϊ���ޱ�Ҫ�� ��
����װ�ñ��ij������¿ڴ�������ͼ(b)��ʾ�Ķ�����ݣ��������ĺô���
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʻ�Ϻ����������ɣ��������г�����������ɵ��ǣ� ��
A��ͭƬ�������Ȼ�����Һ
B�����ۺ�����������Һ
C��ƫ��������Һ������ϡ����
D���������ƺ������Ȼ�������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���ij��ɫ��Һ�������Ӽ���ķ����ͽ�����ȷ����(����)
A������NaOH��Һ�������ɫ��������ԭ��Һ��һ����FeCl3
B���������������ʹ����ʯ��ˮ����ǵ����壬��ԭ��Һ��һ����CO ��SO
��SO
C����ͨ������Cl2���ټ��������Һ����Һ������˵����I��
D������Һ�м���BaCl2��Һ��ϡHNO3���а�ɫ�������ɣ�˵��һ����SO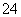
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ʷ���ȫ����ȷ ( )
��С�մ� ��ʳ��ˮ ��ʯ��ˮ ��NaOH ��Һ̬�� ��KClO3
A.��٢� B. �Ρ��٢� C. ������ۢܢ� D.�����ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA��ʾ����٤������������˵����ȷ���ǡ������� �� ��
A��1mol������������ԭ��Ӧ��һ��ת��2NA������
B��1mol����������ԭ��Ӧ��һ��ʧȥ3NA������
C��2.7gH2O����������1.5NA��
D��0.1mol/L��Na2SO4��Һ�У�����������Ϊ0.2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ե��з�̪��Һ��������Һ����������ɫ�������
A��������Һ����
B��CH3COONa��Һ����
C����ˮ�м�������NH4Cl����
D��С�մ���Һ�м�������NaCl����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�������£�CH3COONa��Һ����ˮ��ƽ�⣺CH3COO����H2O CH3COOH��OH��������˵����ȷ����
CH3COOH��OH��������˵����ȷ����
A����������NaOH���壬c(CH3COO��)����
B����������FeCl3���壬c(CH3COO��)����
C��ϡ����Һ����Һ��pH����
D��������������õ������Ի����Һ��c(Na��)��c(CH3COO��)��c(H��)��c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��¯����ʱ�ô�O2����������Ʒ�����ݲ��������ȷ���л������ɡ�����װ������ȼ�շ�ȷ���л������ʽ���õ�װ�á�

(1)������O2�������ҵ�������ѡװ�ø����ܵ���ȷ����˳����_________________________________________��
(2)Cװ����Ũ�����������_____________________________________________��
(3)Dװ����MnO2��������_____________________________________________��
(4)ȼ�չ���CuO��������______________________________________________��
(5)��ʵ������ȡ��Ʒֻ��C��H��O����Ԫ���е����ֻ����֣�ȷ��ȡ0.92 g��Ʒ������ַ�Ӧ��A����������1.76 g��B����������1.08 g�������Ʒ��ʵ��ʽΪ________________��
(6)Ҫȷ�������ʵķ���ʽ����Ҫ֪�������ʵ�____________________�����ⶨ�������ܶ�Ϊ2.054 g��L��1(�ѻ���Ϊ��״����)���������ʽΪ____________��
(7)�����ʵĺ˴Ź���������ͼ��ʾ������ṹ��ʽΪ____________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������Ƿ�����Ԫ�أ���Ҫ��ʵ�鷽����(����)
A��������ˮ������ˮ�����Ƿ����غ�ɫ�������
B��������������Һ���ټ�ϡ���ᣬ�۲�����dz��ɫ��������
C������NaOH��Һ���ȣ���ȴ�����������Һ���۲�����dz��ɫ��������
D������NaOH��Һ���ȣ���ȴ���ù���ϡ�����к����ļ���ټ���������Һ���۲�����dz��ɫ��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com