
ŅŃÖŖōŹ»ł»ÆŗĻĪļÓė±„ŗĶNaHSO3ČÜŅŗæÉŅŌ·¢ÉśŅŌĻĀ·“Ó¦£ŗ
![]()
£Ø1£©ōŹ»ł»ÆŗĻĪļŗĶ±„ŗĶNaHSO3µÄ·“Ó¦ĖŁĀŹČēĻĀ£ŗ
| ōŹ»ł»ÆŗĻĪļ | CH3CHO | CH3COCH3 | C2H5COCH3 | CH3CH2CH2COCH3 |
| ²śĀŹ£Ø1Š”Ź±ÄŚ£© | 88.7 | 56.2 | 36.4 | 23.4 |
| ōŹ»ł»ÆŗĻĪļ | £ØCH3£©2CHCOCH3 | £ØCH3£©3CCOCH3 | C2H5COC2H5 | C6H5COCH3 |
| ²śĀŹ£Ø1Š”Ź±ÄŚ£© | 12.3 | 5.6 | 2 | 1 |
æɼū£¬Č”“ś»ł¶ŌōŹ»ł»ÆŗĻĪļŗĶNaHSO3·“Ó¦µÄÓ°ĻģÓŠ£ØŠ“³ö3Ģõ¼“æÉ£©
¢Ł
¢Ś
¢Ū
£Ø2£©ĄūÓĆŅŌÉĻæÉÄę·“Ó¦æÉŅŌ·ÖĄėČ©ŗĶĶŖµÄ»ģŗĻĪļ£¬ĒėŠ“³öÄÜŹ¹Č©ÓėNaHSO3Éś³ÉµÄ³ĮµķÖŲŠĀČܽāµÄŹŌ¼ĮµÄ»ÆѧŹ½ £ØŠ“³ö2ÖÖ£¬ŹōÓŚ²»Ķ¬Ąą±šµÄĪļÖŹ”££©
£Ø3£©¼ģŃé¼×»łĶŖ£ØRCOCH3£©Ķس£ÓƵ½ĻĀĮŠ2²½·“Ó¦Ą“Éś³ÉÄŃČÜÓŚĖ®µÄĀČ·Ā”£
![]()
¢ŁŠ“³öµŚŅ»²½·“Ó¦µÄ»Æѧ·½³ĢŹ½
¢ŚŠ“³öAµÄ½į¹¹¼ņŹ½
£Ø4£©±½¼×Č©ŌŚÅؼī×÷ÓĆĻĀ·“Ӧɜ³É±½¼×ĖįŃĪŗĶ±½¼×“¼£¬“Ė·“Ó¦µÄĄąŠĶŹĒ ”£
 Ó®ŌŚæĪĢĆĆūŹ¦æĪŹ±¼Ę»®ĻµĮŠ“š°ø
Ó®ŌŚæĪĢĆĆūŹ¦æĪŹ±¼Ę»®ĻµĮŠ“š°ø ĢģĢģĻņÉĻæĪŹ±Ķ¬²½ŃµĮ·ĻµĮŠ“š°ø
ĢģĢģĻņÉĻæĪŹ±Ķ¬²½ŃµĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
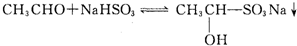
| ōĒ»ł»ÆŗĻĪļ | CH3CHO | CH3COCH3 | C2H3COCH3 | CH3CH2CH2COCH3 |
| ²śĀŹ/% £Ø1Š”Ź±ÄŚ£© |
88.7 | 56.2 | 36.4 | 23.4 |
| ōĒ»ł»ÆŗĻĪļ | £ØCH3£©2CHCOCH3 | £ØCH3£©3CCOCH3 | C2H3COC2H3 | C6H5COCH3 |
| ²śĀŹ/% £Ø1Š”Ź±ÄŚ£© |
12.3 | 5.6 | 2 | 1 |
| l2£¬NaOH |
| NaOH |
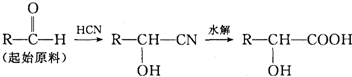
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗĶ¬²½Ģā ĢāŠĶ£ŗĢīæÕĢā
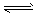
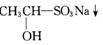

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖōŹ»ł»ÆŗĻĪļÓė±„ŗĶNaHSO3ČÜŅŗæÉŅŌ·¢ÉśŅŌĻĀ·“Ó¦£ŗ
![]()
£Ø1£©ōŹ»ł»ÆŗĻĪļŗĶ±„ŗĶNaHSO3µÄ·“Ó¦ĖŁĀŹČēĻĀ£ŗ
| ōŹ»ł»ÆŗĻĪļ | CH3CHO | CH3COCH3 | C2H5COCH3 | CH3CH2CH2COCH3 |
| ²śĀŹ£Ø1Š”Ź±ÄŚ£© | 88.7 | 56.2 | 36.4 | 23.4 |
| ōŹ»ł»ÆŗĻĪļ | £ØCH3£©2CHCOCH3 | £ØCH3£©3CCOCH3 | C2H5COC2H5 | C6H5COCH3 |
| ²śĀŹ£Ø1Š”Ź±ÄŚ£© | 12.3 | 5.6 | 2 | 1 |
æɼū£¬Č”“ś»ł¶ŌōŹ»ł»ÆŗĻĪļŗĶNaHSO3·“Ó¦µÄÓ°ĻģÓŠ£ØŠ“³ö3Ģõ¼“æÉ£©
¢Ł
¢Ś
¢Ū
£Ø2£©ĄūÓĆŅŌÉĻæÉÄę·“Ó¦æÉŅŌ·ÖĄėČ©ŗĶĶŖµÄ»ģŗĻĪļ£¬ĒėŠ“³öÄÜŹ¹Č©ÓėNaHSO3Éś³ÉµÄ³ĮµķÖŲŠĀČܽāµÄŹŌ¼ĮµÄ»ÆѧŹ½ £ØŠ“³ö2ÖÖ£¬ŹōÓŚ²»Ķ¬Ąą±šµÄĪļÖŹ”££©
£Ø3£©¼ģŃé¼×»łĶŖ£ØRCOCH3£©Ķس£ÓƵ½ĻĀĮŠ2²½·“Ó¦Ą“Éś³ÉÄŃČÜÓŚĖ®µÄĀČ·Ā”£
![]()
¢ŁŠ“³öµŚŅ»²½·“Ó¦µÄ»Æѧ·½³ĢŹ½
¢ŚŠ“³öAµÄ½į¹¹¼ņŹ½
£Ø4£©±½¼×Č©ŌŚÅؼī×÷ÓĆĻĀ·“Ӧɜ³É±½¼×ĖįŃĪŗĶ±½¼×“¼£¬“Ė·“Ó¦µÄĄąŠĶŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com