
| A����KI������Һ��ͨ��Cl2����Һ������˵������������۷�����ɫ��Ӧ |
| B��Ũ�����ڹ��������±��,˵��Ũ����ȶ�,����ɫ����������������Ũ���� |
| C����ij��Һ�м���HNO3�ữ��BaCl2��Һ�а�ɫ��������,˵����Һ�к���SO42- |
| D����ͭƬ����Ũ������������ʵ������˵��ͭ�����Ũ�����з����ۻ� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ�鲽�� | ʵ����� | Ԥ������ͽ��� |
| ��һ�� | | |
| �ڶ��� | | |
| ������ | | |
| ���IJ� | | |
| | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
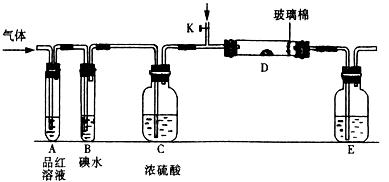
 ��SO2��Cl2��װ��A�й۲쵽�������Ƿ���ͬ�� (���ͬ������ͬ��)����װ��D��װ��
��SO2��Cl2��װ��A�й۲쵽�������Ƿ���ͬ�� (���ͬ������ͬ��)����װ��D��װ�� ��V2O5(����)��ͨ��SO2ʱ����Kͨ������O2�Ļ�ѧ��Ӧ����ʽΪ ��
��V2O5(����)��ͨ��SO2ʱ����Kͨ������O2�Ļ�ѧ��Ӧ����ʽΪ �� �� C���������
�� C��������� Һ D��������Һ
Һ D��������Һ l2�뺬X����Һ��Ӧ�����ӷ���ʽ ��
l2�뺬X����Һ��Ӧ�����ӷ���ʽ ��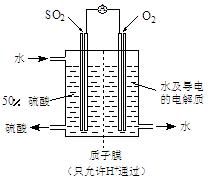

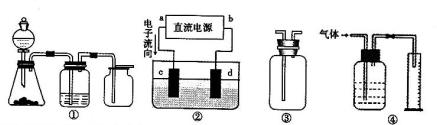
 ��aΪ���⣬dΪ����
��aΪ���⣬dΪ���� 2��NH3��Cl2,��HCl��NO2��
2��NH3��Cl2,��HCl��NO2�� ��װ�â������ڲ����������
��װ�â������ڲ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ���ԭϡ��Һ��I-Ũ�ȣ��ζ�ʱ�ķ�ӦΪ��2Na2S2O3 + I2 = Na2S4O6 + 2NaI���Իش�
���ԭϡ��Һ��I-Ũ�ȣ��ζ�ʱ�ķ�ӦΪ��2Na2S2O3 + I2 = Na2S4O6 + 2NaI���Իش� ____________����ǡ����ǡ�������ΪAgX�����������Ļ�ѧʽΪ__________������ΪAgX��������˿ղ����
____________����ǡ����ǡ�������ΪAgX�����������Ļ�ѧʽΪ__________������ΪAgX��������˿ղ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
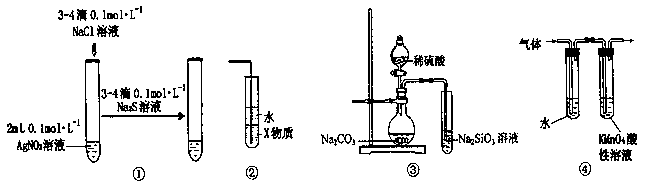
| A��װ�â�����֤AgCl������ת��Ϊ�ܽ�ȸ�С��Ag2S���� |
| B��װ�â���X��Ϊ���Ȼ�̼�����������հ���������ֹ���� |
| C��װ�â۵�ʵ����ƶ���̼��������Ԫ�صķǽ�����ǿ�� |
| D��װ�âܿɼ��������鷢����ȥ��Ӧ�õ��������к�����ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
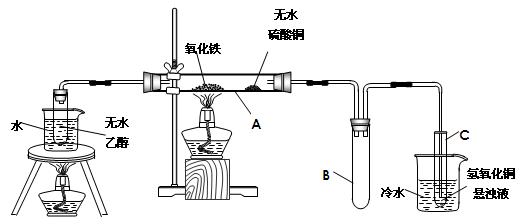
�鿴�𰸺ͽ���>>
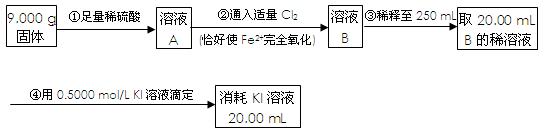
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 Fe2O3+SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺
Fe2O3+SO2��+SO3��+14H2O������ͼ��װ�ÿ���������������Ӧ�����е���������ش��������⣺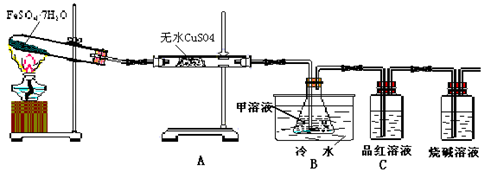
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
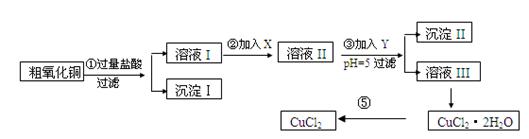
| ���� | ��ʼ����ʱpH | ��ȫ����ʱpH |
| Fe(OH)3 | 2��7 | 3��7 |
| Fe(OH)2 | 7��6 | 9��6 |
| Cu(OH)2 | 5��2 | 6��4 |
| ������ | ����pH������ | ||
| A | ˫��ˮ | D | ��ˮ |
| B | ������� | E | ��ʽ̼��ͭ |
| C | ��ˮ | F | ����ͭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ƽ���������зֱ�ŵ�������ֽ����ȡ2.0g NaOH���� |
| B����NaOH�������ձ����ܽ��Ѹ��С��ת����250mL����ƿ�� |
| C������ʱ��С�ļ�ˮ�����˿̶��ߣ���ʱѸ���ý�ͷ�ι�����һЩ |
| D����Һ������ϣ���������ת�������������Լ�ƿ�����ϱ�ǩ��ϴ������ƿ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com