
ĶŃĮņ¼¼ŹõÄÜÓŠŠ§æŲÖĘSO2¶ŌæÕĘųµÄĪŪČ¾”£
(1)ĻņĆŗÖŠ¼ÓČėŹÆ»ŅŹÆæɼõÉŁČ¼ÉÕ²śĪļÖŠSO2µÄŗ¬Į棬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ____________________________ ”£
(2)ŗ£Ė®³ŹČõ¼īŠŌ£¬Ö÷ŅŖŗ¬ÓŠNa£«”¢K£«”¢Ca2£«”¢Mg2£«”¢Cl£”¢SO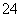 ”¢Br£”¢HCO
”¢Br£”¢HCO µČ”£ŗ¬SO2µÄŃĢĘųæÉĄūÓĆŗ£Ė®ĶŃĮņ£¬Ę乤ŅÕĮ÷³ĢČēĶ¼ĖłŹ¾£ŗ
µČ”£ŗ¬SO2µÄŃĢĘųæÉĄūÓĆŗ£Ė®ĶŃĮņ£¬Ę乤ŅÕĮ÷³ĢČēĶ¼ĖłŹ¾£ŗ

¢ŁĻņĘŲĘų³ŲÖŠĶØČėæÕĘųµÄÄæµÄŹĒ______________________________________________”£
¢ŚĶØČėæÕĘųŗó£¬ĘŲĘų³ŲÖŠµÄŗ£Ė®ÓėĢģČ»ŗ£Ė®Ļą±Č£¬ÅضČÓŠĆ÷ĻŌ²»Ķ¬µÄĄė×ÓŹĒ______(Ģī×ÖÄø)”£
a£®Cl£ b£®SO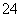 c£®Br£ d£®HCO
c£®Br£ d£®HCO
(3)ÓĆNaOHČÜŅŗĪüŹÕŃĢĘųÖŠµÄSO2£¬½«ĖłµĆµÄNa2SO3ČÜŅŗ½ųŠŠµē½ā£¬æɵƵ½NaOH£¬Ķ¬Ź±µĆµ½H2SO4£¬ĘäŌĄķČēĶ¼ĖłŹ¾”£(µē¼«²ÄĮĻĪŖŹÆÄ«)

¢ŁĶ¼ÖŠa¼«Į¬½ÓµēŌ“µÄ______(Ģī”°Õż”±»ņ”°øŗ”±)¼«£¬CæŚĮ÷³öµÄĪļÖŹŹĒ________”£
¢ŚSO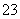 ·ÅµēµÄµē¼«·“Ó¦ĪŖ________________________”£
·ÅµēµÄµē¼«·“Ó¦ĪŖ________________________”£
¢Ūµē½ā¹ż³ĢÖŠŅõ¼«Ēų¼īŠŌĆ÷ĻŌŌöĒ棬ÓĆĘ½ŗāŅĘ¶ÆµÄŌĄķ½āŹĶ¼īŠŌŌöĒæµÄŌŅņ£ŗ________________________________________________________________________”£
“š°ø””(1)2SO2£«O2£«2CaCO3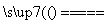 2CaSO4£«2CO2
2CaSO4£«2CO2
(2)¢Ł½«H2SO3”¢HSO µČŃõ»ÆĪŖSO
µČŃõ»ÆĪŖSO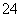 ””¢Śbd
””¢Śbd
(3)¢Łøŗ””ĮņĖį””¢ŚSO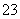 £2e££«H2O===SO
£2e££«H2O===SO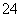 £«2H£«
£«2H£«
¢ŪH2O??H£«£«OH££¬H£«ŌŚŅõ¼«·ÅµēÉś³ÉH2£¬c(H£«)¼õŠ”£¬Ė®µÄµēĄėĘ½ŗāÕżĻņŅĘ¶Æ£¬¼īŠŌŌöĒæ
½āĪö””(1)ĆŗČ¼ÉÕŹ±£¬ŹÆ»ŅŹÆŌŚøßĪĀĻĀ·Ö½ā²śÉśCaOŗĶCO2£¬CaOĪŖ¼īŠŌŃõ»ÆĪļ£¬æÉŅŌÓėSO2”¢O2·“Ӧɜ³ÉCaSO4£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ2SO2£«O2£«2CaCO3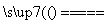 2CaSO4£«2CO2”£(2)SO2ĪŖĖįŠŌŃõ»ÆĪļ£¬ŗ£Ė®³ŹČõ¼īŠŌ£¬ĘŲĘų³ŲÖŠĶØČėæÕĘųµÄÄæµÄŹĒ½«SO
2CaSO4£«2CO2”£(2)SO2ĪŖĖįŠŌŃõ»ÆĪļ£¬ŗ£Ė®³ŹČõ¼īŠŌ£¬ĘŲĘų³ŲÖŠĶØČėæÕĘųµÄÄæµÄŹĒ½«SO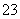 ”¢HSO
”¢HSO µČŃõ»Æ”£ĶØČėæÕĘųŗó£¬ČÜŅŗÖŠSO
µČŃõ»Æ”£ĶØČėæÕĘųŗó£¬ČÜŅŗÖŠSO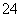 µÄÅضČŌö“ó£¬HCO
µÄÅضČŌö“ó£¬HCO µÄÅØ¶Č¼õŠ””£(3)µē½āNa2SO3ČÜŅŗ£¬øł¾ŻĶ¼Ź¾£¬a¼«Į¬½ÓµēŌ“µÄøŗ¼«£¬CæŚĮ÷³öµÄĪļÖŹŹĒĮņĖį”£Ņõ¼«µÄµē¼«·“Ó¦Ź½ĪŖ2H£«£«2e£===H2”ü£¬Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖSO
µÄÅØ¶Č¼õŠ””£(3)µē½āNa2SO3ČÜŅŗ£¬øł¾ŻĶ¼Ź¾£¬a¼«Į¬½ÓµēŌ“µÄøŗ¼«£¬CæŚĮ÷³öµÄĪļÖŹŹĒĮņĖį”£Ņõ¼«µÄµē¼«·“Ó¦Ź½ĪŖ2H£«£«2e£===H2”ü£¬Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖSO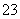 £2e££«H2O===SO
£2e££«H2O===SO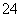 £«2H£«”£µē½ā¹ż³ĢÖŠH£«ŌŚŅõ¼«·ÅµēÉś³ÉH2£¬Ņõ¼«Ēų“ę
£«2H£«”£µē½ā¹ż³ĢÖŠH£«ŌŚŅõ¼«·ÅµēÉś³ÉH2£¬Ņõ¼«Ēų“ę
ŌŚĘ½ŗāH2O??H£«£«OH££¬c(H£«)¼õŠ”£¬Ė®µÄµēĄėĘ½ŗāÕżĻņŅĘ¶Æ£¬¼īŠŌŌöĒ攣


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷Ō×Ó»ņĄė×ӵĵē×ÓÅŲ¼Ź½“ķĪóµÄŹĒ(””””)
A£®C””1s22s22p2
B£®O2£””1s22s22p6
C£®Cr””1s22s22p63s23p63d44s2
D£®Al3£«””1s22s22p6
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŵ±“¶ū»Æѧ½±µĆÖ÷ŌóĪ¬¶ūŃŠ¾æ·¢ĻÖ£¬µ±¼¤¹āĀö³åÕÕÉäNaIŹ±£¬Na£«ŗĶI£Į½ŗĖ¼ä¾ąŌŚ10”«15 £¬³ŹĄė×Ó¼ü£»µ±Į½ŗĖææ½üŌ¼2.8
£¬³ŹĄė×Ó¼ü£»µ±Į½ŗĖææ½üŌ¼2.8 Ź±£¬³Ź¹²¼Ū¼ü”£øł¾ŻŌóĪ¬¶ūµÄŃŠ¾æ³É¹ūÄÜµĆ³öµÄ½įĀŪŹĒ(””””)
Ź±£¬³Ź¹²¼Ū¼ü”£øł¾ŻŌóĪ¬¶ūµÄŃŠ¾æ³É¹ūÄÜµĆ³öµÄ½įĀŪŹĒ(””””)
A£®NaI¾§ĢåŹĒĄė×Ó¾§ĢåŗĶ·Ö×Ó¾§ĢåµÄ»ģŗĻĪļ B£®¹²¼Ū¼üŗĶĄė×Ó¼üƻӊĆ÷ĻŌµÄ½ēĻŽ
C£®NaI¾§ĢåÖŠ¼ČÓŠĄė×Ó¼ü£¬ÓÖÓŠ¹²¼Ū¼ü D£®Ąė×Ó¾§ĢåæÉÄÜŗ¬ÓŠ¹²¼Ū¼ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)ĻĀĮŠĪļÖŹÖŠ£¬æÉŠĪ³ÉĖįÓźµÄŹĒ______”£
A£®¶žŃõ»ÆĮņ B£®·śĀČ“śĢž
C£®¶žŃõ»ÆĢ¼ D£®¼×Ķé
(2)ĻÖÓŠŅŌĻĀ¼øÖÖ“ėŹ©£ŗ¢Ł¶ŌČ¼ÉÕĆŗŹ±²śÉśµÄĪ²Ęų½ųŠŠ³żĮņ“¦Ąķ£»¢ŚÉŁÓĆŌĆŗ×÷Č¼ĮĻ£»¢ŪČ¼ĆŗŹ±¹ÄČė×ćĮææÕĘų£»¢ÜæŖ·¢Ēå½ąÄÜŌ“”£ĘäÖŠÄܼõÉŁĖįÓź²śÉśµÄ“ėŹ©ŹĒ______”£
A£®¢Ł¢Ś¢Ū B£®¢Ś¢Ū¢Ü
C£®¢Ł¢Ś¢Ü D£®¢Ł¢Ū¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¶ŌijĖįŠŌČÜŅŗ(æÉÄÜŗ¬ÓŠBr£”¢SO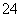 ”¢H2SO3”¢NH
”¢H2SO3”¢NH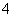 )·Ö±š½ųŠŠČēĻĀŹµŃé£ŗ
)·Ö±š½ųŠŠČēĻĀŹµŃé£ŗ
¢Ł¼ÓČČŹ±·Å³öµÄĘųĢåæÉŅŌŹ¹Ę·ŗģČÜŅŗĶŹÉ«£»
¢Ś¼Ó¼īµ÷ÖĮ¼īŠŌŗ󣬼ÓČČŹ±·Å³öµÄĘųĢåæÉŅŌŹ¹ČóŹŖµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£»
¢Ū¼ÓČėĀČĖ®Ź±£¬ČÜŅŗĀŌĻŌ»ĘÉ«£¬ŌŁ¼ÓČėBaCl2ČÜŅŗ£¬²śÉśµÄ°×É«³Įµķ²»ČÜÓŚĻ”ĻõĖį”£
¶ŌÓŚĻĀĮŠĪļÖŹ²»ÄÜČ·ČĻĘäŌŚŌČÜŅŗÖŠŹĒ·ń“ęŌŚµÄŹĒ(””””)
A£®Br£ B£®SO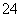 C£®H2SO3 D£®NH
C£®H2SO3 D£®NH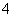
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½¹ŃĒĮņĖįÄĘ(Na2S2O5)ŹĒ³£ÓƵď³Ę·æ¹Ńõ»Æ¼ĮÖ®Ņ»”£Ä³ŃŠ¾æŠ”×é½ųŠŠČēĻĀŹµŃé£ŗ
ŹµŃéŅ»””½¹ŃĒĮņĖįÄʵÄÖĘČ”
²ÉÓĆĻĀĶ¼×°ÖĆ(ŹµŃéĒ°ŅŃ³ż¾”×°ÖĆÄŚµÄæÕĘų)ÖĘČ”Na2S2O5”£×°ÖĆ¢ņÖŠÓŠNa2S2O5¾§ĢåĪö³ö£¬·¢ÉśµÄ·“Ó¦ĪŖNa2SO3£«SO2===Na2S2O5”£
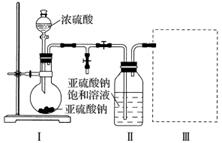
(1)×°ÖĆ¢ńÖŠ²śÉśĘųĢåµÄ»Æѧ·½³ĢŹ½ĪŖ__________________________________________”£
(2)ŅŖ“Ó×°ÖĆ¢ņÖŠ»ńµĆŅŃĪö³öµÄ¾§Ģ壬æɲÉČ”µÄ·ÖĄė·½·ØŹĒ________________________________________________________________________
________________________________________________________________________ӣ
(3)×°ÖĆ¢óÓĆÓŚ“¦ĄķĪ²Ęų£¬æÉŃ”ÓƵÄ×īŗĻĄķ×°ÖĆ(¼Š³ÖŅĒĘ÷ŅŃĀŌČ„)ĪŖ__________(ĢīŠņŗÅ)”£
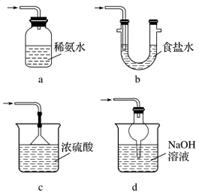
ŹµŃ鶞””½¹ŃĒĮņĖįÄʵĊŌÖŹ
Na2S2O5ČÜÓŚĖ®¼“Éś³ÉNaHSO3”£
(4)Ö¤Ć÷NaHSO3ČÜŅŗÖŠHSO µÄµēĄė³Ģ¶Č“óÓŚĖ®½ā³Ģ¶Č£¬æɲÉÓƵďµŃé·½·ØŹĒ_____(ĢīŠņŗÅ)”£
µÄµēĄė³Ģ¶Č“óÓŚĖ®½ā³Ģ¶Č£¬æɲÉÓƵďµŃé·½·ØŹĒ_____(ĢīŠņŗÅ)”£
a£®²ā¶ØČÜŅŗµÄpH
b£®¼ÓČėBa(OH)2ČÜŅŗ
c£®¼ÓČėŃĪĖį
d£®¼ÓČėĘ·ŗģČÜŅŗ
e£®ÓĆĄ¶É«ŹÆČļŹŌÖ½¼ģ²ā
(5)¼ģŃéNa2S2O5¾§ĢåŌŚæÕĘųÖŠŅѱ»Ńõ»ÆµÄŹµŃé·½°øŹĒ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹö¢ńŗĶŠšŹö¢ņ¾łÕżČ·ĒŅÓŠŅņ¹ū¹ŲĻµµÄŹĒ(””””)
| Ń”Ļī | ŠšŹö¢ń | ŠšŹö¢ņ |
| A | H2ÓŠ»¹ŌŠŌ£¬ÅØĮņĖįÓŠĒæŃõ»ÆŠŌ | ²»ÄÜÓĆÅØĮņĖįøÉŌļH2 |
| B | CuSÄŃČÜÓŚĖ®ŗĶĮņĖį | ·“Ó¦£ŗH2S£«CuSO4===CuS”ż£«H2SO4æÉŅŌ·¢Éś |
| C | ÅØH2SO4ÓŠĪüĖ®ŠŌ | ÅØH2SO4æÉÓĆÓŚøÉŌļ°±Ęų |
| D | SO2¾ßÓŠĖįŠŌŗĶĘư׊Ō | Ķł×ĻÉ«ŹÆČļČÜŅŗÖŠĶØČėSO2£¬ČÜŅŗĻȱäŗģŌŁĶŹÉ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĶ¼ĪŖ¼øÖÖĮ£×ӵĽį¹¹Ź¾ŅāĶ¼£¬Ķź³ÉŅŌĻĀĢīæÕ”£
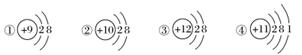
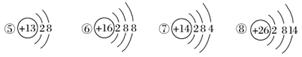
(1)ŹōÓŚŃōĄė×Ó½į¹¹µÄĮ£×ÓŹĒ________(Ģī±ąŗÅ£¬ĻĀĶ¬)”£
(2)¾ßÓŠĪČ¶ØŠŌ½į¹¹µÄŌ×ÓŹĒ__________________”£
(3)Ö»ÄܵƵē×ÓµÄĮ£×ÓŹĒ______________£»Ö»ÄÜŹ§µē×ÓµÄĮ£×ÓŹĒ______________£»¼ČÄܵƵē×Ó£¬ÓÖÄÜŹ§µē×ÓµÄĮ£×ÓŹĒ____________________________”£
(4)¢ŪĮ£×Ó°ė¾¶________¢ÜĮ£×Ó°ė¾¶(Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±)”£
(5)ijŌŖĖŲRŠĪ³ÉµÄŃõ»ÆĪļĪŖR2O3£¬ŌņRµÄĄė×Ó½į¹¹Ź¾ŅāĶ¼æÉÄÜŹĒ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĶÓėŅ»¶ØĮæÅØĻõĖį·“Ó¦£¬µĆµ½ĻõĖįĶČÜŅŗŗĶNO2”¢N2O4”¢NOµÄ»ģŗĻĘųĢ壬ÕāŠ©ĘųĢåÓė5.6 L O2(±ź×¼×“æö)»ģŗĻŗóĶØČėĖ®ÖŠ£¬ĖłÓŠĘųĢåĶźČ«±»Ė®ĪüŹÕÉś³ÉĻõĖį”£ŌņĻūŗÄĶµÄÖŹĮæĪŖ(””””)
A£®16 g B£®32 g
C£®64 g D£®ĪŽ·Ø¼ĘĖć
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com