
����Ԫ��A��B��C��D����ԭ�ӽṹ�������Ϣ���±���
Ԫ�� �����Ϣ
A Aԭ�ӵĺ�����������������̬���Ӳ���
B Bԭ�ӵ������������Ǵ�����������2��
C C�Ļ�̬ԭ��L���Ӳ�����3��δ�ɶԵ���
D D����Χ���Ӳ��Ų�Ϊ(n+1)d3n(n+2)sn
��ش��������⣺
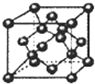
(1)A��ԭ�ӽṹʾ��ͼ��_______��DԪ��λ�����ڱ���______���� ______�塣
(2)����B��A�γɵ��ڶ�����У�ֻҪBԭ�ӵijɼ��������______(����ĸ��ţ������������е�ԭ�Ӿ��п�����ͬһ��ƽ���ڡ�
A��sp3 ��sp2 �ӻ����
B��sp3 ��sp �ӻ����
C��sp ��sp2 �ӻ����
(3)��ͼ����B�����һ�������к���______��Bԭ�ӡ�

(4)�Ʊ�C2A4�ķ������ô�������(NaClO)��Һ����������CA3��
��CA3������______(����ԡ��Ǽ��ԡ������ӡ�
��д���Ʊ�C2A4���ܷ�Ӧ����ʽ____________
(5)DC13��������ˮ��Һ�����ԣ���ԭ����(�����ӷ���ʽ��ʾ����______________________________
��16�֣�
��1�� ��2�֣����� ��1�֣� �� ��1�֣�
��2�֣����� ��1�֣� �� ��1�֣�
��2��C ��2�֣�
��3��8 ��2�֣�
��4���ټ��� ��2�֣� ��NaClO+2NH3=N2H4+NaCl+H2O ��3�֣�
��5��Fe3++3H2O Fe(OH)3+3H+
��3�֣�
Fe(OH)3+3H+
��3�֣�
��������
������������������֪A��B��C��D�ֱ�ΪH��C��N��Fe��
��2��sp2Ϊƽ��ṹ��sp3Ϊֱ���ͽṹ���ʾ����棬��ΪA
��3����ͼ�п��Կ�����C��λ�������֣��ֱ�Ϊ���㡢���ġ����ģ�Ϊ8�� +6��
+6��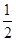 +4��1=8
+4��1=8
��4��NH3Ϊ�����Σ���Ϊ���Է��ӣ����ݷ�Ӧ��Ͳ�����������ԭ��Ӧ��ƽ�ķ��������Եõ���
��3��FeCl3Ϊǿ�������Σ�ˮ������ԡ�
���㣺�����ʽṹΪ������������ԭ�ӽṹ�����ӽṹ������ṹ��������ԭ��Ӧ������ˮ������֪ʶ��


 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | �����Ϣ |
| A | Aԭ�ӵĺ�����������������̬���Ӳ��� |
| B | Bԭ�ӵ������������Ǵ�����������2�� |
| C | C�Ļ�̬ԭ��L���Ӳ�����3��δ�ɶԵ��� |
| D | D����Χ���Ӳ��Ų�Ϊ��n+1��d3n��n+2��sn |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08������ģ�⣩��15�֣������ڳ���Ԫ��A��B��C��D��ԭ��������������
�� C�����������������ڲ��������һ����
�� A��B�γɵĻ�����X��B������������Ӧ��ˮ����Y���������Ϸ�Ӧ����Z��
�� ���C��D�γɵĻ������ˮ��Һ���������ų���������ͬ���������������ȡ�
�Իش�
��DΪ ����Ԫ�ط��ţ���X�ĵ���ʽΪ ��
��Z��ˮ��Һ�� ������ԡ������ԡ������ԡ���
�����ӷ���ʽ˵��ԭ��
�ǵ����C��D�γɵĻ������������ˮ��Һ500mlʱ����������������1120ml����״����������Һ��PH= ��������Һ������䣬�¶�Ϊ���£�
��A���ʺ�B������һ�������·�����Ӧ������ʼ����n A��n B=1��1ʱ��A���ʵ�ת��
�� B���ʵ�ת���ʣ��������������=����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com