
| A£®0”«10minÄŚv£ØH2£©=0.3mol/£ØL?min£© | ||
B£®T”ꏱ£¬Ę½ŗā³£ŹżK=
| ||
| C£®T”ꏱ£¬ÉĻŹö·“Ó¦ÖŠÓŠ64gCH3OHÉś³É£¬Ķ¬Ź±·Å³ö98.0kJµÄČČĮæ | ||
| D£®“ļµ½Ę½ŗāŗó£¬ÉżøßĪĀ¶Č»ņŌŁ³äČėCO2ĘųĢ壬¶¼æÉŅŌĢįøßH2µÄ×Ŗ»ÆĀŹ |

| ||
| 1L |
| 1”Į1 |
| 1”Į33 |
| 1 |
| 27 |
| 64g |
| 32g/mol |


 ŗģ¹ū×ÓČż¼¶²āŹŌ¾ķĻµĮŠ“š°ø
ŗģ¹ū×ÓČż¼¶²āŹŌ¾ķĻµĮŠ“š°ø æĪĢĆĮ·¼Ó²āĻµĮŠ“š°ø
æĪĢĆĮ·¼Ó²āĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø2010?³ÆŃōĒųÄ£Äā£©T”ꏱ£¬ŌŚ1LµÄĆܱÕČŻĘ÷ÖŠ³äČė2mol CO2ŗĶ6mol H2£¬Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗCO2£Øg£©+3H2£Øg£©=CH3OH£Øg£©+H2O£Øg£©”÷H=-49.0kJ/mol²āµĆH2ŗĶCH3OH£Øg£©µÄÅضČĖꏱ¼ä±ä»ÆČēĶ¼ĖłŹ¾£®ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©
£Ø2010?³ÆŃōĒųÄ£Äā£©T”ꏱ£¬ŌŚ1LµÄĆܱÕČŻĘ÷ÖŠ³äČė2mol CO2ŗĶ6mol H2£¬Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗCO2£Øg£©+3H2£Øg£©=CH3OH£Øg£©+H2O£Øg£©”÷H=-49.0kJ/mol²āµĆH2ŗĶCH3OH£Øg£©µÄÅضČĖꏱ¼ä±ä»ÆČēĶ¼ĖłŹ¾£®ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø2011?Šū³Ē¶žÄ££©T”ꏱ£¬ŌŚ1LµÄĆܱÕČŻĘ÷ÖŠ³äČė2mol CO2ŗĶ6mol H2£¬Ņ»¶ØĢõ¼žĻĀ·¢Éś·“Ó¦£ŗCO2£Øg£©+3H2£Øg£©?CH3OH£Øg£©+H2O£Øg£©£¬”÷H=-49.0kJ?mol-1²āµĆH2ŗĶCH3OH£Øg£©µÄÅضČĖꏱ¼ä±ä»ÆČēĻĀĶ¼ĖłŹ¾£®ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©
|
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŠū³Ē¶žÄ£ ĢāŠĶ£ŗµ„Ń”Ģā
| Ź±¼ä | c £ØH2£©/mol?L-1 | c £ØCH3OH£©/mol?L-1 | v£ØÕż£©ŗĶv £ØÄę£© ±Č½Ļ |
| t0 | 6 | 0 | £æ |
| t1 | 3 | 1 | v£ØÕż£©=v£ØÄę£© |
A£®t0”«t1Ź±¼äÄŚ¦Ō£ØH2£©=
| ||
| B£®t1Ź±£¬ČōÉżøßĪĀ¶Č»ņŌŁ³äČėCO2ĘųĢ壬¶¼æÉŅŌĢįøßH2µÄ×Ŗ»ÆĀŹ | ||
| C£®t0Ź±£¬v£ØÕż£©£¾v£ØÄę£© | ||
| D£®T”ꏱ£¬Ę½ŗā³£ŹżK=1/27£¬CO2ÓėH2µÄ×Ŗ»ÆĀŹĻąµČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź±±¾©ŹŠ³ÆŃōĒųøßæ¼»ÆŃ§Ä£ÄāŹŌ¾ķ£Ø¶ž£©£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā

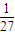 £¬CO2ŗĶH2µÄ×Ŗ»ÆĀŹĻąµČ
£¬CO2ŗĶH2µÄ×Ŗ»ÆĀŹĻąµČ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com