
��ȩ��Һ���û�����ֲ������ϲ�Ϊ��ɫ��״Һ�壬�²�Ϊˮ��Һ������ �����ϲ�����Ϊ��ȩ�ľۺ���(CH2O)![]() ���÷�������ȩ�����ҷе��ˮ�ߡ���֪��(CH2O)n��nCH2O(���Ի�����)
���÷�������ȩ�����ҷе��ˮ�ߡ���֪��(CH2O)n��nCH2O(���Ի�����)
(1)��ȩ��Һ���ڿ������ױ�������֤����ȩ�ѱ�������ʵ�������������
��
(2)��������ͼa��ʾװ����ȡ��ȩ����ƿװ(CH2O)n��6mol��Lϡ������Һ�����������ڣ�(CH2O)n�ֽ⣬�����ļ�ȩ���屻��ƿ�е�����ˮ���գ��������ܵĽ�ˮ����Ϊ (�a����b��)�����������л�����Һ���� ��������δ������ƿҺ���µ�ԭ���� ��
�����¼�ȩ�ж������°�����»�����֮һ���ҹ��涨�����ҿ����еļ�ȩ���������Ũ��Ϊ0.08mg��m3��ij��ѧ�о�С�������·����ⶨ�����еļ�ȩ��Ũ��(���������������ԭ������)��
�ⶨԭ�������Ը�����ؿ�������ȩ�Ͳ��ᣬ�����ӷ���ʽΪ��
4MnO![]() +5HCHO+12H+==4Mn2++5CO2��+11H2O
+5HCHO+12H+==4Mn2++5CO2��+11H2O
( )MnO![]() +( )H2C2O4+( )H+��( )Mn2++( )CO2��+( )H2O
+( )H2C2O4+( )H+��( )Mn2++( )CO2��+( )H2O
�ⶨװ����ͼb��ʾ��
ʵ�鲽�裺
(3)���װ�õ������ԣ���μ��װ�õ�������?
��
(4)�� (����������)ȡ�������Ը��������Һװ����ƿ�С�
(5)��a���ر�b����ע������ȡ100 mL��װ�����ڵĿ������ر�a����b���ٻ����ƶ�ע������������ȫ���������Ը��������Һ�У�ʹ���ַ�Ӧ��������ظ���Σ����ѹ��������ٶȹ��죬�Բⶨ������Ӱ��?
��

(6)�ò���ζ����ƿ�е���Һ��(�Ҫ����Ҫ��) ָʾ�����յ��ʵ�������� ����������ص�Ũ��Ϊc1�����ΪV1mL�������Ũ��Ϊc2�����IJ�����Һ��ƽ�����ΪV2 mL�����ȩ��Ũ���� mg��m3��(���ȡ�Ŀ������Ϊ100mL)
(1)ȡ�����²�ˮ��Һ���μ�ʯ����Һ������Һ��죬˵����ȩ�ѱ�����
(2)b H2O(����������ȩ) ��ȩ������ˮ����(����Ȳ�����)��������
(3)���ڹ��ƿ��װˮ��û�������¶ˣ��ر�a����b�������ƶ�ע�������������¶�������ð����������ע�������������¶�����һ��ˮ����˵������������
(4)��ʽ�ζ���
(5)��ѹ��������ٶȹ��죬��ȩ���������Ը��������Һ��ַ�Ӧ��ʹ�ⶨ���ƫ��
(6)��Ҫ ��ƿ����Һ����ɫ�����ɫ�Ұ�����ڲ���ɫ�� 30��(c1V1һ0��4c2V2)��10һ3��5��4��103��(0��1��10һ3)mg��m3 ��ʾ����ƽ����ʽ����㣬2MnO![]() +5H2C2O4+6H+==2Mn2++10CO2��+8H2O��
+5H2C2O4+6H+==2Mn2++10CO2��+8H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
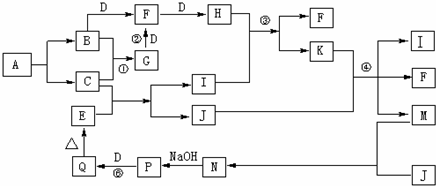
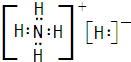
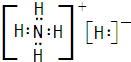
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
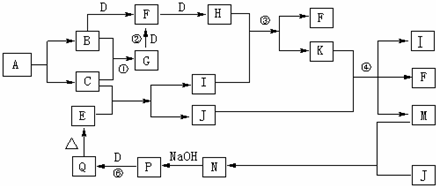
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com