
 ���� ��˵����Ӧ���ȡ�������װ����֧�ż��������������ȥ��
���� ��˵����Ӧ���ȡ�������װ����֧�ż��������������ȥ��
 ��
��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| װ�ñ�� | A | B | C | D | |
| װ�� | 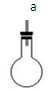 |  |  | 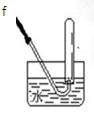 | |
| װ���������� | | | | | |
| �������Ⱥ��������,ʵ��װ������˳��͵������ӷ��� | װ������˳�� ����װ�ñ�ţ� | ||||
| ��Ҫ���ȵ�װ�� | ����װ�ñ�ţ� | ||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
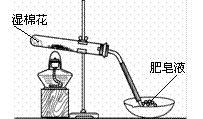
| �μӵ���Һ | ��ˮ | ��ˮ |
| �����Ļ�ѧʽ | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��Cu(OH)2��м�ʽ����ͭ���ɱ�ʾΪxCuSO4.yCu(OH)2��.Ϊ�˼�����֤��С���Ա����������ʵ�飺
��Cu(OH)2��м�ʽ����ͭ���ɱ�ʾΪxCuSO4.yCu(OH)2��.Ϊ�˼�����֤��С���Ա����������ʵ�飺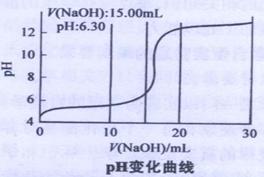
| A����ͷ�ι� | B����ʽ�ζ��� | C����ʽ�ζ��� | D����Ͳ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
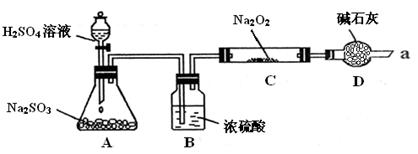

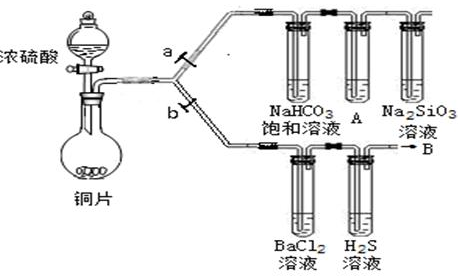
| ʵ�鲽�� | ʵ������ |
| �ٵμ��������ϡ���� | �����ݼ�����ζ���� |
| �ڵμ���������BaCl2��Һ | ������ɫ������ |
| ��ȡ����C�й���������Թ��У���������������ˮ�ܽ� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �������� ��
�������� ��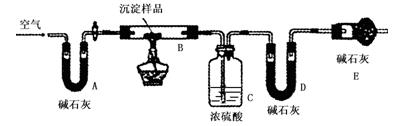
| | Bװ�õ�������g�� | Cװ�õ�������g�� | Dװ�õ�������g�� |
| ʵ��ǰ | 15.4 | 262.1 | 223.8 |
| ����� | 6.1 | 264.8 | 230.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A�����Ҵ�����ͨ�� ���ȵ��� ���ȵ��� ��ͭ��������ɫ�ɺ�ɫ��Ϊ��ɫ ��ͭ��������ɫ�ɺ�ɫ��Ϊ��ɫ |
| B������һ���¶ȡ�ѹǿ�ʹ����������º�������Ӧ�����ɻ����� |
| C��һ�������£���������Ũ�����Ũ����Ļ��Һ�У�����״������ |
| D������ˮ�м������Ȼ�̼��Һ��ˮ����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ����� | ����ͽ��� |
| ����һ��ȡ��ȡ����ϲ���Һ�μ�2��K4[Fe(CN)6] | ��_________�������һ�������� |
| ���������̽����������Һ�м��������� �ѣ���������÷ֲ� | �����Ѳ��Ѫ��ɫ����___________�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com