
��ͭ�Ͻ�㷺���ں��չ�ҵ�����и�����л��������Ʊ�ͭ������Ʒ�Ĺ�������ͼ��
��1����⾫����ʱ��������ӦʽΪ_______________������A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���ú���ɫ������ˮ��Ӧ�Ļ�ѧ����ʽΪ_______________________________________��
��2����������B�����Ϊ_____________�������ɹ���B�Ĺ����У��������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ_____________________��
��3�����չ��������ɵ�����������NH3�ڴ��������·�Ӧ�Ļ�ѧ����ʽΪ_____________________�������Ӧ�л��а��̲������ð���Ϊ______________��
��4������ͭ�Ͻ���ͭ����������Ϊ64����������3��0kg�����е�ͭ����ȫת��Ϊ__________molCuAlO2��������Ҫ1��0 mol��L��1��Al2(SO4)3��Һ___________L��
( ��10�֣�(1)Ag+ + e����Ag ��1�֣� 3NO2 +H2O��2HNO3 + NO�� (1�֣�
��2��CuO����Cu(OH)2�� ��Al(OH)3��1�֣� OH�D +Al(OH)3��AlO2-+2H2O��1�֣�
��3��4NH3+5O2 4NO+6H2O��1�֣� NH4NO3��1�֣� (4)30 ��1�֣���15 ��1�֣�
4NO+6H2O��1�֣� NH4NO3��1�֣� (4)30 ��1�֣���15 ��1�֣�
���������������1�����վ���ͭ��ԭ������ȷ��������������Ag��e���� Ag+��������������Ag++e���� Ag������A�еĽ�����ϡ���ᷴӦ������ɫ��NO��NO������е�������Ӧ���ɺ���ɫ��NO2��NO2��ˮ��Ӧ���������NO����Ӧ�Ļ�ѧ����ʽΪ3NO2 +H2O��2HNO3 + NO����
��2�������Ϣ������ͼ������֪������ͭ��������������ϡ�������Ʒ�Ӧ����������ͭ���������������ʱ������ͭ�ֽ�ΪCuO�������������ֽ⣬����BӦ��ΪCuO��Al(OH)3���������������������������������NaOH��������������������Al(OH)3�ͻ��ܽ⣬��Ӧ�����ӷ���ʽΪAl(OH)3+OH����AlO��+2H2O��
��3���ڴ����������£�������������������NO��ˮ����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2 4NO+6H2O�����ɵ�NO��������ˮ���������������ᣬ�����백����Ӧ��������臨�ð���̡�
4NO+6H2O�����ɵ�NO��������ˮ���������������ᣬ�����백����Ӧ��������臨�ð���̡�
��4����ͭ�Ͻ��е�ͭ�����ʵ���n(Cu)�� ��30mol������Ԫ���غ�ɵ����ɵ�CuAlO2Ҳ��30.0mol�����ݻ�ѧʽCuAlO2�е�Cu��Al������ϵ��Alԭ�Ӹ����غ�ɵ�,n[Al2(SO4)3]�� 30.0mol��2��15.0mol��������Ҫ��������Һ�������15.0mol��1.0mol/L��15.0L��
��30mol������Ԫ���غ�ɵ����ɵ�CuAlO2Ҳ��30.0mol�����ݻ�ѧʽCuAlO2�е�Cu��Al������ϵ��Alԭ�Ӹ����غ�ɵ�,n[Al2(SO4)3]�� 30.0mol��2��15.0mol��������Ҫ��������Һ�������15.0mol��1.0mol/L��15.0L��
���㣺������ԭ����Ӧ�á������Ʊ���ʵ�鷽������������Լ����㡢�����Ĵ�������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ������п����Ҫ�ɷ�ΪZnS��������Fe2O3�����ʣ�Ϊԭ������ZnSO4��7H2O�Ĺ����������£�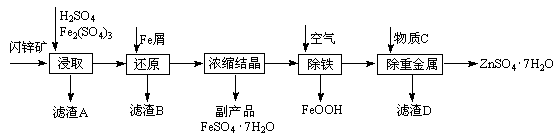
��1��������A�пɻ��һ�ֵ���ɫ�ǽ������ʵĸ���Ʒ���仯ѧʽΪ ��
��2����ȡ������Fe2(SO4)3�������� ����ȡʱFe2(SO4)3��ZnS������Ӧ�Ļ�ѧ����ʽΪ ��
��3���������̿�����Һ��pH��5.4���ң��÷�Ӧ�����ӷ���ʽΪ ���ù����ڿ�����ڴ������һ��������ԡ��ͷ��װ�ã���Ŀ���� ��
��4���û������ؽ���������������CΪ ��
��5������п���ܽ�����¶�֮��Ĺ�ϵ���±���
| �¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
| �ܽ��/g | 41.8 | 54.1 | 70.4 | 74.8 | 67.2 | 60.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ�����ĩ,���п��ܺ���Na2CO3��NaCl��Na2SO4��Ba(NO3)2��K2CO3��K2SO4�е�һ�ֻ��֣��ְ����в������ʵ�顣
��1�����÷�ĩ����ˮ����ɫ��Һ�Ͱ�ɫ������
��2�����˳��ij����м���ϡ���ᣬ�в��ֳ����ܽ⣬ͬʱ������ɫ���塣
��3��ȡ��Һ����ɫ��Ӧ����֤����Һ�к�Na+������K+��
�����������ƶϣ�
��1���û������һ������ ���� ��һ�������� �����ܺ��� ��
��2����Ҫ�Կ��ܺ��е����ʽ��м��飬��β�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ƿ��ǩ�������ɫ����Һ���ֱ���ϡ���ᡢϡ�����ϡ���ᡣ�������ʵ����ʦ������ַ����������ǡ�
| | �� �� �� �� | �� �� �� �� �� |
| ����һ | | |
| ������ | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ﮣ�LiFePO4������Ϊ������ǰ;������ӵ���������ϡ�ij��ҵ���ø�������Һ����������ﮣ������˴�������������Һһ����;��������Ҫ�������£�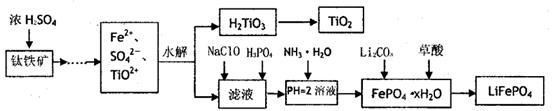
��֪��H2LiO3����������ˮ�����ʡ�
(1)��������Ũ���ᴦ��֮ǰ����Ҫ���飬��Ŀ��
(2)TiO2+ˮ������H2TiO3�����ӷ���ʽ
(3)����NaClO������Ӧ�����ӷ���ʽ
(4)��ʵ���У�����Һ�й��˳�H2TiO3��������Һ���ǣ�Ӧ��β��� ��
(5)Ϊ�ⶨ�����������ĺ�����ijͬѧȡ��Ũ����ȴ�������Һ����ʱ�������е�����ȫ��ת��Ϊ���������ӣ�����ȡKMnO4��Һ�춨Fe2+�ķ�����(������KMnO4���������ʷ�Ӧ)�ڵζ������У���δ�ñ�Һ��ϴ�ζ��ܣ���ʹ�ⶨ��� �� (�ƫ�ߡ�ƫ�͡���Ӱ�족)���ζ��յ������ ���ζ�����ʱ����ȡa g������������cmol/LKMnO4��Һ�ζ�������VmL������Ԫ�ص����������ı���ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��пʪ��ұ�������У����������ͭ��������Ҫ����Zn��Cd��Fe��Cu�ȣ���ֱ�Ӷ�������ɻ�����Ⱦ��Ҳ����Դ�˷ѣ�����Ϊ���մ���ͭ�����Ʊ�����п����Ĺ�ҵ���̣�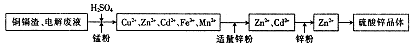
��֪������п����������ˮ�������ھƾ���
��1�����������Ŀ�� ��
��2��������ܳ�ͭ�������̷���Ϊ�˽�������ӵı�������ݵ��ӣ���ͭ�ܳ����̷۵���Ҫ�ɷ�ΪMnO2����ط���ʽΪ2Fe3++Cu=2Fe2++Cu2+�� ��
��3��������п��Һ�������п���壬��Ҫ�IJ�������Ϊ ��
��4���Ƶõ�����п������Ҫϴ�ӣ�ѡ����Լ�Ϊ ��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��ҵ�ϳ����õ�Ʒλ���̿�(��Ҫ�ɷ���MnO2)�����պ�SO2�ķ����������Ƶ������̾���(MnSO4��H2O)������Ҫ�������£�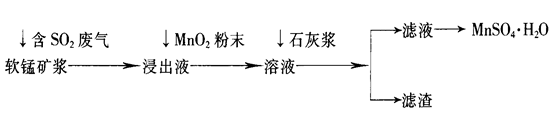
��֪������Һ��pH��2�����еĽ���������Ҫ��Mn2+��������������Fe2+��Al3+�������������ӡ��йؽ��������γ������������ʱ��Һ��pH���±���
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 7.6 | 9.7 |
| Fe3+ | 2.7 | 3.7 |
| Al3+ | 3.8 | 4.7 |
| Mn2+ | 8.3 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(6�֣�A��B��C��D���ֿ����Ի�����ֱ���������Fe3+��Ba2+��Al3+��Na+��������OH-��SO42-��Cl-��CO32-�еĸ�һ����ɣ����Ӳ��ظ�����ͨ��ʵ�飬�ó����½��ۣ�
��A��D����Һ�Լ��ԣ�0��1mol��L-1A��Һ��pHС��13��
��ͭ�����ܽ���B����Һ�У�
����C����Һ�м��������D��Һ������û�г�����
��������ʵ����ʵ���ش��������⣺
��1�� ��д��A��B�Ļ�ѧʽ��A ��B___________��
��2�� ��д��ʵ����з�Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ͭ���ȷֽ���������ͭ�����壬�����¶Ȳ�ͬ������ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO2��SO3��O2�е�һ�֡����ֻ����֡�ij��ѧ����С��ͨ�����̽����ʵ�飬�ⶨ��Ӧ������SO2��SO3��O2�����ʵ�����������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ���õ�����������ͼ��ʾ��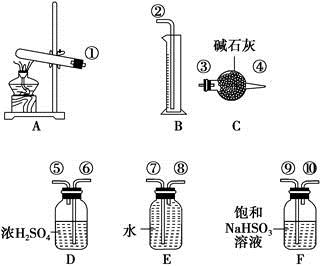
[�������]
��.��������ijɷֿ���ֻ��SO3һ�֣�
��.��������ijɷֿ��ܺ��� ���֣�
��.��������ijɷֿ��ܺ��� ���֡�
[ʵ��̽��]
ʵ����������ԡ���֪ʵ�����ʱ������ͭ��ȫ�ֽ⡣
(1)������װ̽��ʵ���װ�ã����������ҵķ��������ӿڵ�����˳��Ϊ�١��������ޡ��ݡ� �� �� �� ����(��ӿ����)��
(2)��ʵ�����ʱB����Ͳû���ռ���ˮ����֤������ ��ȷ��
(3)������ʵ��С����и�ʵ�飬���ڼ���ʱ���¶Ȳ�ͬ��ʵ����������������Ҳ��ͬ���������£�
| ʵ��С�� | ��ȡCuSO4������/g | װ��C���ӵ�����/g | ��Ͳ��ˮ���������ɱ�״������������/mL |
| һ | 6.4 | 2.56 | 448 |
| �� | 6.4 | 2.56 | 224 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com