
ŹµŃéŹŅ“Óŗ¬µā·ĻŅŗ(³żH2OĶā£¬ŗ¬ÓŠCCl4”¢I2”¢I£µČ)ÖŠ»ŲŹÕµā£¬Ę䏵Ńé¹ż³ĢČēĻĀ£ŗ
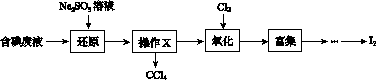
(1)Ļņŗ¬µā·ĻŅŗÖŠ¼ÓČėÉŌ¹żĮæµÄNa2SO3ČÜŅŗ£¬½«·ĻŅŗÖŠµÄI2»¹ŌĪŖI££¬ĘäĄė×Ó·½³ĢŹ½ĪŖ__________________£»øĆ²Ł×÷½«I2»¹ŌĪŖI£µÄÄæµÄŹĒ______________________”£
(2)²Ł×÷XµÄĆū³ĘĪŖ________”£
(3)Ńõ»ÆŹ±£¬ŌŚČż¾±ÉÕĘæÖŠ½«ŗ¬I£µÄĖ®ČÜŅŗÓĆŃĪĖįµ÷ÖĮpHŌ¼ĪŖ2£¬»ŗĀżĶØČėCl2£¬ŌŚ40 ”ę×óÓŅ·“Ó¦(ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾)”£
ŹµŃéæŲÖĘŌŚ½ĻµĶĪĀ¶ČĻĀ½ųŠŠµÄŌŅņŹĒ______________£»×¶ŠĪĘæĄļŹ¢·ÅµÄČÜŅŗĪŖ________”£

(4)ŅŃÖŖ£ŗ5SO £«2IO
£«2IO £«2H£«===I2£«5SO
£«2H£«===I2£«5SO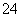 £«H2O
£«H2O
ijŗ¬µā·ĻĖ®(pHŌ¼ĪŖ8)ÖŠŅ»¶Ø“ęŌŚI2£¬æÉÄÜ“ęŌŚI£”¢IO ÖŠµÄŅ»ÖÖ»ņĮ½ÖÖ”£Ēė²¹³äĶźÕū¼ģŃéŗ¬µā·ĻĖ®ÖŠŹĒ·ńŗ¬ÓŠI£”¢IO
ÖŠµÄŅ»ÖÖ»ņĮ½ÖÖ”£Ēė²¹³äĶźÕū¼ģŃéŗ¬µā·ĻĖ®ÖŠŹĒ·ńŗ¬ÓŠI£”¢IO µÄŹµŃé·½°ø£ŗČ”ŹŹĮæŗ¬µā·ĻĖ®ÓĆCCl4¶ą“ĪŻĶČ””¢·ÖŅŗ£¬Ö±µ½Ė®²ćÓƵķ·ŪČÜŅŗ¼ģŃé²»³öÓŠµāµ„ÖŹ“ęŌŚ£»________________________________________________________________________
µÄŹµŃé·½°ø£ŗČ”ŹŹĮæŗ¬µā·ĻĖ®ÓĆCCl4¶ą“ĪŻĶČ””¢·ÖŅŗ£¬Ö±µ½Ė®²ćÓƵķ·ŪČÜŅŗ¼ģŃé²»³öÓŠµāµ„ÖŹ“ęŌŚ£»________________________________________________________________________
________________________________________________________________________
________________________________________________________________________ӣ
ŹµŃéÖŠæɹ©Ń”ŌńµÄŹŌ¼Į£ŗĻ”ŃĪĖį”¢µķ·ŪČÜŅŗ”¢FeCl3ČÜŅŗ”¢Na2SO3ČÜŅŗ”£
(1)SO £«I2£«H2O===2I££«SO
£«I2£«H2O===2I££«SO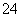 £«2H£«””Ź¹CCl4ÖŠµÄµā½ųČėĖ®²ć
£«2H£«””Ź¹CCl4ÖŠµÄµā½ųČėĖ®²ć
(2)·ÖŅŗ
(3)Ź¹ĀČĘųŌŚČÜŅŗÖŠÓŠ½Ļ“óµÄČܽā¶Č(»ņ·ĄÖ¹I2Éż»Ŗ»ņ·ĄÖ¹I2½ųŅ»²½±»Ńõ»Æ)””NaOHČÜŅŗ
(4)“ÓĖ®²ćȔɣĮæČÜŅŗ£¬¼ÓČė1”«2 mLµķ·ŪČÜŅŗ£¬¼ÓŃĪĖįĖį»Æ£¬µĪ¼ÓFeCl3ČÜŅŗ£¬ČōČÜŅŗ±äĄ¶£¬ĖµĆ÷·ĻĖ®ÖŠŗ¬ÓŠI££»ČōČÜŅŗ²»±äĄ¶£¬ĖµĆ÷·ĻĖ®ÖŠ²»ŗ¬ÓŠI£”£Įķ“ÓĖ®²ćȔɣĮæČÜŅŗ£¬¼ÓČė1”«2 mLµķ·ŪČÜŅŗ£¬¼ÓŃĪĖįĖį»Æ£¬µĪ¼ÓNa2SO3ČÜŅŗ£¬ČōČÜŅŗ±äĄ¶£¬ĖµĆ÷·ĻĖ®ÖŠŗ¬ÓŠIO £»ČōČÜŅŗ²»±äĄ¶£¬ĖµĆ÷·ĻĖ®ÖŠ²»ŗ¬ÓŠIO
£»ČōČÜŅŗ²»±äĄ¶£¬ĖµĆ÷·ĻĖ®ÖŠ²»ŗ¬ÓŠIO
[½āĪö] (1)SO ±»I2Ńõ»ÆĪŖSO
±»I2Ńõ»ÆĪŖSO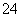 £¬I2±»»¹ŌĪŖI££¬½įŗĻµēŗÉŹŲŗćŗĶŌ×ÓŹŲŗćæɵĆSO
£¬I2±»»¹ŌĪŖI££¬½įŗĻµēŗÉŹŲŗćŗĶŌ×ÓŹŲŗćæɵĆSO £«I2£«H2O===2I££«SO
£«I2£«H2O===2I££«SO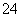 £«2H£«”£ŅņĪŖI2²»ČÜÓŚĖ®£¬¶ųµā»ÆĪļŅ×ČÜÓŚĖ®£¬¹Ź½«I2»¹ŌĪŖI£µÄÄæµÄŹĒŹ¹µāŌŖĖŲ½ųČėĖ®²ć”£(2)·ÖĄėÓŠ»śČܼĮŗĶĖ®ČÜŅŗµÄ»ģŗĻĪļŠčŅŖ·ÖŅŗ”£(3)ĪĀ¶ČŌ½øߣ¬Cl2Čܽā¶ČŌ½Š”£¬¶ųĒŅĖęĪĀ¶ČÉżøߣ¬Cl2»į°ŃI2½ųŅ»²½Ńõ»ÆĪŖIO
£«2H£«”£ŅņĪŖI2²»ČÜÓŚĖ®£¬¶ųµā»ÆĪļŅ×ČÜÓŚĖ®£¬¹Ź½«I2»¹ŌĪŖI£µÄÄæµÄŹĒŹ¹µāŌŖĖŲ½ųČėĖ®²ć”£(2)·ÖĄėÓŠ»śČܼĮŗĶĖ®ČÜŅŗµÄ»ģŗĻĪļŠčŅŖ·ÖŅŗ”£(3)ĪĀ¶ČŌ½øߣ¬Cl2Čܽā¶ČŌ½Š”£¬¶ųĒŅĖęĪĀ¶ČÉżøߣ¬Cl2»į°ŃI2½ųŅ»²½Ńõ»ÆĪŖIO £¬µ¼ÖĀŃõ»ÆI£µÄŠ§ĀŹĘ«µĶ£»ĮķĶā£¬I2Ņ²ČŻŅ×Éż»Ŗ”£
£¬µ¼ÖĀŃõ»ÆI£µÄŠ§ĀŹĘ«µĶ£»ĮķĶā£¬I2Ņ²ČŻŅ×Éż»Ŗ”£
(4)¼ģŃéI2ÓƵķ·Ū£¬½įŗĻĖłøųŹŌ¼ĮµÄŠŌÖŹ£¬FeCl3¾ßÓŠŃõ»ÆŠŌ£¬æɽ«I£Ńõ»ÆĪŖI2£¬¶ųNa2SO3¾ßÓŠĒ滹ŌŠŌ£¬æɽ«IO »¹ŌĪŖI2”£
»¹ŌĪŖI2”£


 Č«Óųå“Ģ100·ÖĻµĮŠ“š°ø
Č«Óųå“Ģ100·ÖĻµĮŠ“š°ø Ó¢²Åµć½ņĻµĮŠ“š°ø
Ó¢²Åµć½ņĻµĮŠ“š°ø ŗģ¹ū×ÓČż¼¶²āŹŌ¾ķĻµĮŠ“š°ø
ŗģ¹ū×ÓČż¼¶²āŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹ŲČÜŅŗ×é³ÉµÄĆčŹöŗĻĄķµÄŹĒ(””””)
A£®ĪŽÉ«ČÜŅŗÖŠæÉÄÜ“óĮæ“ęŌŚAl3£«”¢NH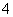 ”¢Cl£”¢S2£
”¢Cl£”¢S2£
B£®ĖįŠŌČÜŅŗÖŠæÉÄÜ“óĮæ“ęŌŚNa£«”¢ClO£”¢SO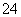 ”¢I£
”¢I£
C£®Čõ¼īŠŌČÜŅŗÖŠæÉÄÜ“óĮæ“ęŌŚNa£«”¢K£«”¢Cl£”¢HCO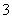
D£®ÖŠŠŌČÜŅŗÖŠæÉÄÜ“óĮæ“ęŌŚFe3£«”¢K£«”¢Cl£”¢SO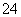
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
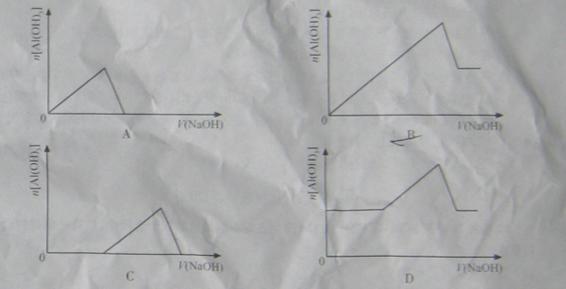
ŅŃÖŖŹŅ ĪĀĻĀ£¬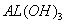 µÄK£¬»ņČܽā¶ČŌ¶“óÓŚ
µÄK£¬»ņČܽā¶ČŌ¶“óÓŚ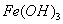 ”£ĻņÅØ¶Č¾łĪŖ0.1
”£ĻņÅØ¶Č¾łĪŖ0.1

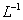 µÄ
µÄ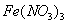 ŗĶ
ŗĶ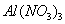 »ģŗĻČÜŅŗÖŠ£¬ÖšµĪ¼ÓČėNaOH ČÜŅŗ”£ĻĀĮŠŹ¾ŅāĶ¼±ķŹ¾Éś³É
»ģŗĻČÜŅŗÖŠ£¬ÖšµĪ¼ÓČėNaOH ČÜŅŗ”£ĻĀĮŠŹ¾ŅāĶ¼±ķŹ¾Éś³É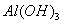 µÄĪļÖŹµÄĮæÓė¼ÓČėNaOHČÜŅŗµÄĢå»żµÄ¹ŲĻµ ”£ŗĻĄķµÄŹĒ
µÄĪļÖŹµÄĮæÓė¼ÓČėNaOHČÜŅŗµÄĢå»żµÄ¹ŲĻµ ”£ŗĻĄķµÄŹĒ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹÆÄ«ŌŚ²ÄĮĻĮģÓņÓŠÖŲŅŖÓ¦ÓĆ”£Ä³³õ¼¶ŹÆÄ«ÖŠŗ¬SiO2(7.8%)”¢Al2O3(5.1%)”¢Fe2O3(3.1%)ŗĶMgO(0.5%)µČŌÓÖŹ”£Éč¼ĘµÄĢį“æÓė×ŪŗĻĄūÓĆ¹¤ŅÕČēĻĀ£ŗ
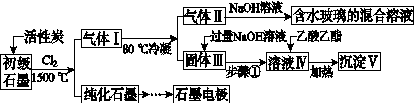
(×¢£ŗSiCl4µÄ·ŠµćĪŖ57.6 ”ę£¬½šŹōĀČ»ÆĪļµÄ·Šµć¾łøßÓŚ150 ”ę)
(1)Ļņ·“Ó¦Ę÷ÖŠĶØČėCl2Ē°£¬ŠčĶØŅ»¶ĪŹ±¼äN2£¬Ö÷ŅŖÄæµÄŹĒ____________________”£
(2)øßĪĀ·“Ó¦ŗó£¬ŹÆÄ«ÖŠŃõ»ÆĪļŌÓÖŹ¾ł×Ŗ±äĪŖĻąÓ¦µÄĀČ»ÆĪļ”£ĘųĢå¢ńÖŠµÄĢ¼Ńõ»ÆĪļÖ÷ŅŖĪŖ________”£ÓÉĘųĢå¢ņ֊ijĪļµĆµ½Ė®²£Į§µÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ____________________________________________”£
(3)²½Öč¢ŁĪŖ£ŗ½Į°č”¢________”£ĖłµĆČÜŅŗ¢ōÖŠµÄŅõĄė×ÓÓŠ________”£
(4)ÓÉČÜŅŗ¢ōÉś³É³Įµķ¢õµÄ×Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ______________________________________________£¬100 kg³õ¼¶ŹÆÄ«×ī¶ąæÉÄÜ»ńµĆ¢õµÄÖŹĮæĪŖ______kg”£
(5)ŹÆÄ«æÉÓĆÓŚ×ŌČ»Ė®ĢåÖŠĶ¼žµÄµē»Æѧ·ĄøÆ£¬Ķź³ÉČēĶ¼·ĄøÆŹ¾ŅāĶ¼£¬²¢×÷ĻąÓ¦±ź×¢”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
LiPF6ŹĒļ®Ąė×Óµē³ŲÖŠ¹ć·ŗÓ¦ÓƵĵē½āÖŹ”£Ä³¹¤³§ÓĆLiF”¢PCl5ĪŖŌĮĻ£¬µĶĪĀ·“Ó¦ÖʱøLiPF6£¬ĘäĮ÷³ĢČēĻĀ£ŗ
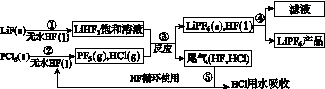
ŅŃÖŖ£ŗHClµÄ·ŠµćŹĒ£85.0 ”ę£¬HFµÄ·ŠµćŹĒ19.5 ”ę”£
(1)µŚ¢Ł²½·“Ó¦ÖŠĪŽĖ®HFµÄ×÷ÓĆŹĒ________________”¢________________”£·“Ó¦Éč±ø²»ÄÜÓĆ²£Į§²ÄÖŹµÄŌŅņŹĒ______________________________________________(ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾)”£ĪŽĖ®HFÓŠøÆŹ“ŠŌŗĶ¶¾ŠŌ£¬¹¤³§°²Č«ŹÖ²įĢįŹ¾£ŗČē¹ū²»Š”ŠÄ½«HFÕ“µ½Ę¤·ōÉĻ£¬æÉĮ¢¼“ÓĆ2%µÄ________ČÜŅŗ³åĻ“”£
(2)øĆĮ÷³ĢŠčŌŚĪŽĖ®Ģõ¼žĻĀ½ųŠŠ£¬µŚ¢Ū²½·“Ó¦ÖŠPF5¼«Ņ×Ė®½ā£¬Ęä²śĪļĪŖĮ½ÖÖĖį£¬Š“³öPF5Ė®½āµÄ»Æѧ·½³ĢŹ½£ŗ____________________________________”£
(3)µŚ¢Ü²½·ÖĄė²ÉÓƵķ½·ØŹĒ________£»µŚ¢Ż²½·ÖĄėĪ²ĘųÖŠHF”¢HCl²ÉÓƵķ½·ØŹĒ________”£
(4)LiPF6²śĘ·ÖŠĶس£»ģÓŠÉŁĮæLiF”£Č”ѳʷw g£¬²āµĆLiµÄĪļÖŹµÄĮæĪŖn mol£¬ŌņøĆѳʷ֊LiPF6µÄĪļÖŹµÄĮæĪŖ________mol(ÓĆŗ¬w”¢nµÄ“śŹżŹ½±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌĀĒņŗ¬ÓŠH”¢He”¢N”¢Na”¢Mg”¢SiµČŌŖĖŲ£¬ŹĒČĖĄąĪ“Ą“µÄ׏Ō“±¦æā”£
(1)3HeŹĒøߊ§ŗĖÄÜŌĮĻ£¬ĘäŌ×ÓŗĖÄŚÖŠ×ÓŹżĪŖ________”£
(2)NaµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼ĪŖ________£¬NaŌŚŃõĘųÖŠĶźČ«Č¼ÉÕĖłµĆ²śĪļµÄµē×ÓŹ½ĪŖ________”£
(3)MgClŌŚ¹¤ŅµÉĻÓ¦ÓĆ¹ć·ŗ£¬æÉÓÉMgOÖʱø”£
¢ŁMgOµÄČŪµć±ČBaOµÄČŪµć________(Ģī”°øß”±»ņ”°µĶ”±)”£
¢ŚŌĀĒņÉĻijæóŹÆ¾“¦ĄķµĆµ½µÄMgOÖŠŗ¬ÓŠÉŁĮæSiO2£¬³żČ„SiO2µÄĄė×Ó·½³ĢŹ½ĪŖ__________________________£»SiO2µÄ¾§ĢåĄąŠĶĪŖ________”£
¢ŪMgOÓėĢæ·ŪŗĶĀČĘųŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦æÉÖʱøMgCl2”£ČōĪ²ĘųæÉÓĆ×ćĮæNaOHČÜŅŗĶźČ«ĪüŹÕ£¬ŌņÉś³ÉµÄŃĪĪŖ________________(Š“»ÆѧŹ½)”£
(4)ŌĀČĄÖŠŗ¬ÓŠ·įø»µÄ3He£¬“ÓŌĀČĄÖŠĢįĮ¶1 kg 3He£¬Ķ¬Ź±æɵĆ6000 kg H2ŗĶ700 kg N2£¬ČōŅŌĖłµĆH2ŗĶN2ĪŖŌĮĻ¾Ņ»ĻµĮŠ·“Ó¦×ī¶ąæÉÉś²śĢ¼ĖįĒāļ§________kg”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ģś¼°Ęä»ÆŗĻĪļÓėÉś²ś”¢Éś»ī¹ŲĻµĆÜĒŠ”£
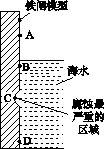
(1)ĻĀĶ¼ŹĒŹµŃéŹŅŃŠ¾æŗ£Ė®¶ŌĢśÕ¢²»Ķ¬²æĪ»øÆŹ“ĒéæöµÄĘŹĆęŹ¾ŅāĶ¼”£
¢ŁøƵē»ÆøÆŹ“³ĘĪŖ________”£
¢ŚĶ¼ÖŠA”¢B”¢C”¢DĖÄøöĒųÓņ£¬Éś³ÉĢśŠā×ī¶ąµÄŹĒ________(Ģī×ÖÄø)”£
(2)ÓĆ·ĻĢśĘ¤ÖĘČ”Ģśŗģ(Fe2O3)µÄ²æ·ÖĮ÷³ĢŹ¾ŅāĶ¼ČēĻĀ£ŗ
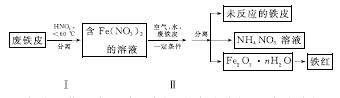
¢Ł²½Öč¢ńČōĪĀ¶Č¹żøߣ¬½«µ¼ÖĀĻõĖį·Ö½ā”£ĻõĖį·Ö½āµÄ»Æѧ·½³ĢŹ½ĪŖ______________________________”£
¢Ś²½Öč¢ņÖŠ·¢Éś·“Ó¦£ŗ4Fe(NO3)2£«O2£«(2n£«4)H2O===2Fe2O3”¤nH2O£«8HNO3£¬·“Ó¦²śÉśµÄHNO3ÓÖ½«·ĻĢśĘ¤ÖŠµÄĢś×Ŗ»ÆĪŖFe(NO3)2£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ____________________________”£
¢ŪÉĻŹöÉś²śĮ÷³ĢÖŠ£¬ÄÜĢåĻÖ”°ĀĢÉ«»Æѧ”±Ė¼ĻėµÄŹĒ______(ČĪŠ“Ņ»Ļī)”£
(3)ŅŃÖŖt ”ꏱ£¬·“Ó¦FeO(s)£«CO(g)Fe(s)£«CO2(g)µÄĘ½ŗā³£ŹżK£½0.25”£
¢Łt ”ꏱ£¬·“Ó¦“ļµ½Ę½ŗāŹ±n(CO)”Ćn(CO2)£½________”£
¢ŚČōŌŚ1 LĆܱÕČŻĘ÷ÖŠ¼ÓČė0.02 mol FeO(s)£¬²¢ĶØČėx mol CO, t ”ꏱ·“Ó¦“ļµ½Ę½ŗā”£“ĖŹ±FeO(s)×Ŗ»ÆĀŹĪŖ50%£¬Ōņx£½________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖĆ¾ŗĶĻ”ĻõĖį·“Ó¦Ź±£¬ĆæÓŠ1 molHNO3 ·“Ó¦£¬¾ĶÓŠ0.8molµē×Ó×ŖŅĘ£¬“ĖŹ±ĻõĖįµÄ»¹Ō²śĪļæÉÄÜŹĒ £Ø £©
A£®NO2 B£®N2O C£®N2O3 D£®NO
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠČÜŅŗÖŠ£¬ČÜÖŹµÄĪļÖŹµÄĮæÅضČĪŖ1 mol•L£1µÄŹĒ( )
A.½«40 g NaOHČÜÓŚ1 LĖ®ÖŠĖłµĆµÄČÜŅŗ
B.½«22.4 L HClČÜÓŚĖ®Åä³É1 LČÜŅŗ
C.ŗ¬K£«µÄĪļÖŹµÄĮæĪŖ2 molµÄK2SO4ČÜŅŗ1 L
D.½«0.5 mol•L£1µÄNaNO3ČÜŅŗ100 mL¼ÓČČÕō·¢µō50 gĖ®µÄČÜŅŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com