
��ˮ����ͭ��ǿ���»ᷢ���ֽⷴӦ��
CuSO4  CuO + SO3�� 2SO3
CuO + SO3�� 2SO3  2SO2��+ O2��
2SO2��+ O2��
����ͼ��ʾװ�ã��г���������ȥ��������D���ڷ�Ӧǰ��������������ֽ��˵���ˮ����ͭ��������
ʵ�鲽�裺
�ٳ�����ӦǰD�ܵ�������
�����Ӻ�װ�ã��ر�K������Ӳ�ʲ�����Aһ��ʱ���ֹͣ���ȡ�
�۴�Ӳ�ʲ�����A��ȴ��K��ͨ��һ��ʱ����ѳ�ȥ������̼����������Ŀ�����
���ٳ���D�ܣ����䷴Ӧǰ���������Ϊm��
�ش��������⣺
��1����Ӧ2SO3(g)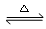 2SO2(g) + O2(g)��ƽ�ⳣ������ʽΪK= ��
2SO2(g) + O2(g)��ƽ�ⳣ������ʽΪK= ��
��2��B���г��¶����������⣬���ɿ����������� �����¶��������ߵ���Ҫԭ���� ��B���з�����Ӧ���й����ӷ���ʽ�� ��
��3������E�������� ��
��4������������ʵ�飬����B��C��D����������վ���ȫ�������Կ�����CO2��Ӱ�죬�ܷ����m������ֽ��˵���ˮCuSO4��������(��ѡ��һ�ش�)
������ܣ���ֽ����ˮCuSO4������Ϊ ����m��ʾ����
��������ܣ���ԭ���� ��Ϊ���ܲ�÷ֽ��˵���ˮ����ͭ����������ļ�ʵ�鷽���� ��
��13�֣���1��K=c(O2)��c2(SO2) / c2(SO3) ��1�֣�
(2) ������ð����������ɫ������2�֣� SO3����ˮ���ȣ�2�֣�
SO3 + H2O + Ba2��= BaSO4��+ 2H��
��SO3 + H2O = 2H��+ SO42����Ba2��+ SO42��= BaSO4����2�֣�
��3�����տ����е�ˮ������CO2��2�֣�
��4����SO3������ȫ�ֽ�ΪSO2��O2��SO2�Ჿ���ܽ�����Һ�У�2�֣�
����װ����ˮ����ͭ��A��������ǿ��һ��ʱ�����ȴ���ٳ���A������������A���ڷ�Ӧǰ��������������ֽ��˵���ˮ����ͭ��������2�֣�
���������������1��ƽ�ⳣ������������Ũ����֮�����Է�Ӧ��Ũ����֮����������ʹ�Һ�岻��д�����ʽ����2����ˮ����ͭ���ȷֽ�ų�SO3��SO2��O2�Ļ�����壬װ��B�з�����ӦSO3+H2O=H2SO4��H2SO4+BaCl2=BaSO4��+2HCl��ǰ�߷��ȣ����߲�����ɫ����SO2��O2���ܷ�Ӧ����װ��B���������ݳ����ܷ�ӦʽΪSO3 + H2O + Ba2��= BaSO4��+ 2H������3����ʯ����NaOH��CaO��ɵĻ��������ն�����̼��ˮ����ֹ���ǽ���װ��D���ų������е�ˮ�����Ͷ�����̼�ĸ��ţ���4�����ܸ���װ��D�ڷ�Ӧǰ���������m������ˮ����ͭ�ֽ��˵�������ԭ���ǣ���SO3������ȫ�ֽ�ΪSO2��O2����SO2�Ჿ���ܽ�����Һ�У���ȷ�����dz���װ����ˮ����ͭ��A��������ǿ��һ��ʱ�����ȴ���ٳ���A������������A���ڷ�Ӧǰ��������������ֽ��˵���ˮ����ͭ��������
���㣺���黯ѧʵ�鷽������������ۣ��漰ƽ�ⳣ������ʽ����д��ʵ�����������������ԭ�����ӷ���ʽ����д����������;��ʵ�鷽������������Ƶȿ��㡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������(NH2COONH4)��һ�ְ�ɫ���壬�ֽ⡢��ˮ�⣬���������ϡ�������ϴ�Ӽ��ȡ�ij��ѧ��ȤС��ģ�ҵԭ���Ʊ���������泥���Ӧ�Ļ�ѧ����ʽ���£�2 NH3(g)+CO2(g)  NH2COONH4(s) ��H��0
NH2COONH4(s) ��H��0 
��1��ʵ�����Ʊ�NH3�Ļ�ѧ����ʽ�ǣ� ��
��2���Ʊ���������淋�װ������ͼ��ʾ���Ѱ����Ͷ�����̼ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ��������С�������������Ȼ�̼�С���������϶�ʱ��ֹͣ�Ʊ���
ע�����Ȼ�̼��Һ��ʯ����Ϊ���Խ��ʡ�
�ٷ������ñ�ˮ��ȴ��ԭ���� ��Һ��ʯ������ƿ�������� ��
�ڴӷ�Ӧ��Ļ�����з������Ʒ��ʵ�鷽���� (��д��������)��Ϊ�˵õ������Ʒ��Ӧ��ȡ�ķ����� (��дѡ�����)��
a. ��ѹ���Ⱥ�� b. ��ѹ���Ⱥ�� c. ���40 �����º��
��β������װ������ͼ��ʾ��˫ͨ�����ܵ����ã� ��
Ũ��������ã� �� �� ����������������
��3��ȡ�ֱ��ʶ�����̼����淋İ����������Ʒ0.7825 g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ�����������Ϊ1.000 g������Ʒ�а�������淋����ʵ�������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧС��Ϊ�ⶨ�ӵ�����KIO3����������������������ʵ�顣
��֪��KIO3 + 5KI + 3H2SO4�� 3K2SO4 + 3I2 + 3H2O
I2 + 2Na2S2O3 �� Na2S4O6 + 2NaI
����һ��ȷ��ȡa g�ӵ��Σ����Ƴ�250mL��Һ��
�������ȡ��������Һ25.00mL����ƿ�У���ϡ�����ữ���ټ���������KI��Һ��
����������bmol��L��1 Na2S2O3��Һ����Һ�ζ������������Һ���յ㣬��¼���ݣ����ظ��ζ�2�Σ�ƽ������Na2S2O3��Һ�����Ϊ12.00mL��
��1������һ������250mL��Һ�����õ��IJ����������ձ����������ͽ�ͷ�ι��⣬���� ��
��2���������н��еζ��Ĺ����������ĸ�������ȷ ��
ѡ�� ��Ϊָʾ��������ζ��յ�ʱ������Ϊ ��
��3��ʵ���ô˼ӵ�����KIO3������������ ��KIO3����Է�������Ϊ214����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��1��С���������о��¶ȶԷ�Ӧ���ʵ�Ӱ�족ʵ��ʱ����ȡ����ֻ�Թܣ�������4mL 0.01mol��L��KMnO4������Һ��2mL 0.1mol/L H2C2O4���Ҷ��ᣩ��Һ����A�Թ�������ˮ�У�B�Թ�������ˮ�У���¼��Һ��ɫ�����ʱ�䡣
����Ҫ�� ���ữKMnO4��Һ����ɫ����ʱ��tA tB���>������=����<������
��д���÷�Ӧ�����ӷ���ʽ ��
��2��ʵ������ƿ������ɳ���Ҷ�����Ʒ��С�����������Ӧ��ԭ�����ⶨ�京�����������Ϊ��
������250 mL��Һ��ȷ����5.0g�Ҷ�����Ʒ�����250mL��Һ��
�ڵζ���ȷ��ȡ25.00 mL������Һ����ƿ�У����������ữ����0.1000 mol��L��1 KMnO4��Һװ�� �����ʽ����ʽ�����ζ��ܣ����еζ�������
��ʵ���з��֣��յ�������KMnO4��Һʱ����ҺѸ�ٱ���Ϻ�ɫ������ƿҡ��һ��ʱ����Ϻ�ɫ������ʧ���ټ����μ�ʱ���Ϻ�ɫ�ͺܿ���ɫ�ˡ������ԭ��
����____
��֤���ﵽ�ζ��յ㡣
�ۼ��㣺���ظ���������2�Σ���¼ʵ���������¡�
| ��� | �ζ�ǰ������mL�� | �ζ��������mL�� |
| 1 | 0.00 | 20.10 |
| 2 | 1.00 | 20.90 |
| 3 | 1.10 | 21.10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧ��ȤС�����������ʵ��װ��(ͼ�в��ּг���������ȥ�����ⶨij��̼�Ͻ�������������������̽������Ũ����ķ�Ӧ��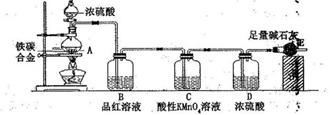
��ش��������⣺
��1��mg��̼�Ͻ��м������Ũ���ᣬδ��ȼ�ƾ���ǰ��A��B�����������裬��ԭ���Ǣ�_____��_____
��2����ȼ�ƾ��ƣ��ɹ۲쵽B�е�������_____________________��C ��������___________________
��3����A�в����ݳ�����ʱ��ֹͣ���ȣ�����E�����أ�E����bg������̼�Ͻ���������������Ϊ________________(д����ʽ)��
��4����ȤС��ʹ����Ƶ���ͼʵ��װ�ã�������ȷ����ÿһ����Ӧ����ȫ����Ȼ��������õ���̼�Ͻ���������������ƫ�ͣ�����Ҫԭ����________________��
��5������Ӧһ��ʱ����õι���ȡA�е���Һ���뵽����ˮ����Ϊ�����������������������ӵijɷ����������ֿ��ܣ�
I��ֻ����Fe3+�� II��ֻ����Fe2+��III��________________________
��֤III��ʵ�鷽����________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��1�����һ����һ�������ʵ���װ�ã���֤���ᡢ������̼ˮ��Һ��̼�ᣩ�ͱ��ӵ����ԣ���ǿ����˳���ǣ�CH3COOH> H2CO3> C6H5OH
��������������������װʵ��װ�ã�������������˳���ǣ� ��
��д��ʵ������з�����Ӧ��ʵ������ �� ��
��2��CO2����Ȼ��ѭ��ʱ����CaCO3��Ӧ��CaCO3��һ���������ʣ���Ksp=2.8��10��9��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ2��10��4mo1/L �������ɳ�������CaCl2��Һ����СŨ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ��ʾΪ���������Ʊ������롢�����������֤�IJ�������װ�ã������豸���г̶ֹ�װ�þ���ȥ���������Ҫ��������и��⣨����װ�ÿ�����ѡ�ã���Ҫʱ���ظ�ѡ�ᡢ��Ϊ��������
��1�����������ͨ��CO��CO2�Ļ�����壬���ڷ���CuO��ѡ��װ�û�ô��������CO������֤�仹ԭ�Լ����������ѡװ�õ�����˳��Ϊ������������������ţ�������֤CO���������������������������������������
��2��ֹͣCO��CO2��������ͨ�룬���ڷ���Na2O2����A��E��D��B��Hװ��˳����ȡ���������O2������O2�����Ҵ�����ʱ������aӦ����������������bӦ����������Ҫ���ȵ�����װ��������������������ţ������з�Ӧ�Ļ�ѧ����ʽΪ����������������
��3����������ڸ�ͨ��������Һ©���ڸļ�Ũ��ˮ��Բ����ƿ�ڸļ�NaOH���壬���ڷ��ò���Ͻ�������A��G��E��Dװ��˳����ȡ����İ���������֤����ijЩ���ʡ�
��װ��A���ܲ���������ԭ��������������������������
��ʵ���й۲쵽E���к���ɫ������֣�֤�������������������ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵����ȷ��
| A�����ع��͡������ܻ��������ڱ��������������ʣ������ڸ߷��ӻ�������Խ����к� |
| B��������ˮʱ�����˻�ѧ�������仯������ɱ�������������� |
| C�������Ѿƴ���ʱ�䳤���������Ϊ�Ҵ�������������Ӧ |
| D�����ơ����Ƚ���Ԫ�ص�������Ѥ������ɫ��������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ijУѧ��������ͼ��ʾװ�ý���ʵ�飬��̽�������巢����Ӧ��ԭ���������ᴿ��Ӧ�IJ��
��ش��������⣺
��1��д��II�з�Ӧ�Ļ�ѧ����ʽ ��
��2���۲쵽II�е������� ��
��3��ʵ�鿪ʼʱ���ر�K2������K1�ͷ�Һ©���������μӱ���Һ��Ļ��Һ����Ӧ��ʼ��III��С�Թ��ڱ��������� ��
��4����˵������Һ�巢����ȡ����Ӧ�������� ��
��5����������ƿ�ڷ�Ӧ���Һ�����ν�������ʵ������Ϳɵõ��ϴ������屽��
��������ˮϴ�ӣ�����Һ������5%��NaOH��Һϴ�ӣ�����Һ��
��������ˮϴ�ӣ�����Һ���ܼ�����ˮCaCl2��ĩ���
�� ����������ƣ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com