
��18�֣���֪��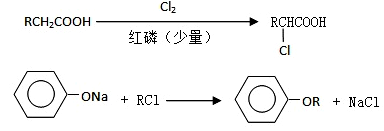
I������ƽF�ǽ�Ѫ֬�������̴���ҩ�����һ���ϳ�·�����£�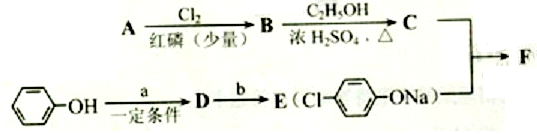
��1��AΪ����һԪ���ᣬ8.8g A������NaHCO3��Һ��Ӧ����2.24L CO2����״������A�ķ���ʽΪ___________________��
��2��д������A����ʽ�����м������Ľṹ��ʽ�� ______________________________��
��3��B���ȴ����ᣬ��˴Ź��������������壬д��B��C�ķ�Ӧ����ʽ��
__________________________________________________________��
��4��C+E��F�ķ�Ӧ����Ϊ________________________��
��5��д��A��F�Ľṹ��ʽ��A______________________�� F__________________________��
��6��D�ı������������⣬�����������ŵ�����Ϊ___________________��д��a��b���������Լ���a ______________; b___________��
��. ������·�ߣ���C�ɺϳɸ߾���H��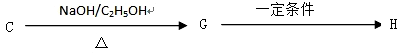
��7��C G�ķ�Ӧ����Ϊ_____________________.
G�ķ�Ӧ����Ϊ_____________________.
��8��д��G H�ķ�Ӧ����ʽ��_______________________��
H�ķ�Ӧ����ʽ��_______________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�I������ƽF�ǽ�Ѫ֬�������̴���ҩ�����һ���ϳ�·�����£�
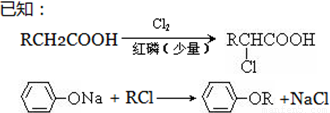
(ע��R��ʾ������Hԭ��)
�� AΪ���ᣬ�����ʽΪC4H8O2��8.8gA������NaHCO3��Һ��Ӧ���� L CO2����״����������CO2����ˮ����
��д������A����ʽ���������ʵĽṹ��ʽ���� ��
����֪A��B����ȡ����Ӧ��BΪһ±�����ᣬ��˴Ź�������ֻ�������壬д��B��C�ķ�Ӧ����ʽ���������� ���������������� ��������
![]() ��д��F�Ľṹ��ʽ������������������������������
��д��F�Ľṹ��ʽ������������������������������
II��������·�ߣ���C�ɺϳ�����߾���H��
C G H
��д��G��H�ķ�Ӧ����ʽ������������������������������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ��У�����ڶ���������ѧ�Ծ� ���ͣ������
��12�֣�I������ƽF�ǽ�Ѫ֬�������̴���ҩ�����һ���ϳ�·�����£�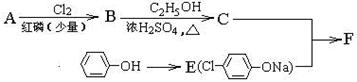
 (ע��R��ʾ������Hԭ��)
(ע��R��ʾ������Hԭ��)
�� AΪ���ᣬ�����ʽΪC4H8O2��8.8gA������NaHCO3��Һ��Ӧ���� L CO2����״����������CO2����ˮ����
��д������A����ʽ���������ʵĽṹ��ʽ���� ��
����֪A��B����ȡ����Ӧ��BΪһ±�����ᣬ��˴Ź�������ֻ�������壬д��B��C�ķ�Ӧ����ʽ����������  ��������������������
��������������������
��д��F�Ľṹ��ʽ������������������������������
II��������·�ߣ���C�ɺϳ�����߾���H��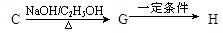
��д��G��H�ķ�Ӧ����ʽ����������������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�߶���ѧ�ڽ�ѧ������ѧ�Ծ��������棩 ���ͣ������
��18�֣���֪��
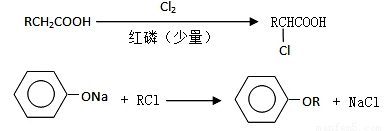
I������ƽF�ǽ�Ѫ֬�������̴���ҩ�����һ���ϳ�·�����£�

��1��AΪ����һԪ���ᣬ8.8g A������NaHCO3��Һ��Ӧ����2.24L CO2����״������A�ķ���ʽΪ___________________��
��2��д������A����ʽ�����м������Ľṹ��ʽ�� ______________________________��
��3��B���ȴ����ᣬ��˴Ź��������������壬д��B��C�ķ�Ӧ����ʽ��
__________________________________________________________��
��4��C+E��F�ķ�Ӧ����Ϊ________________________��
��5��д��A��F�Ľṹ��ʽ��A______________________�� F__________________________��
��6��D�ı������������⣬�����������ŵ�����Ϊ___________________��д��a��b���������Լ���a ______________; b___________��
��. ������·�ߣ���C�ɺϳɸ߾���H��
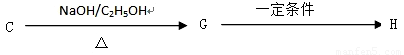
��7��C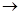 G�ķ�Ӧ����Ϊ_____________________.
G�ķ�Ӧ����Ϊ_____________________.
��8��д��G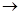 H�ķ�Ӧ����ʽ��_______________________��
H�ķ�Ӧ����ʽ��_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ������У�����ڶ���������ѧ�Ծ� ���ͣ������
��12�֣�I������ƽF�ǽ�Ѫ֬�������̴���ҩ�����һ���ϳ�·�����£�
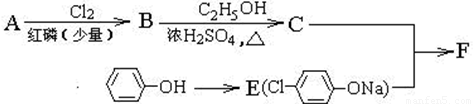
(ע��R��ʾ������Hԭ��)
�� AΪ���ᣬ�����ʽΪC4H8O2��8.8gA������NaHCO3��Һ��Ӧ���� L CO2����״����������CO2����ˮ����
��д������A����ʽ���������ʵĽṹ��ʽ���� ��
����֪A��B����ȡ����Ӧ��BΪһ±�����ᣬ��˴Ź�������ֻ�������壬д��B��C�ķ�Ӧ����ʽ���������� ���������������� ��������
 ��д��F�Ľṹ��ʽ����������������������������
��
��д��F�Ľṹ��ʽ����������������������������
��
II��������·�ߣ���C�ɺϳ�����߾���H��
C
G H
H
��д��G��H�ķ�Ӧ����ʽ���������������������� ��������������������������������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com