
���� ������һԪ����Q��ǿ���������¿ɷֽ������������X��Y��Z������Z��һ�ִ����к����ܸߵ����壬��ҵ�ϳ��÷���Һ̬��������ã���֪ZΪN2��16.5g��Q����������ʵ�����AgBrǡ�÷�Ӧ����������������Ӧ�����õ�54g����MΪAg�������ʵ���Ϊ$\frac{54g}{108g/mol}$=0.5mol����Q����Է�������Ϊ$\frac{16.5}{0.5}$=33����ϣ�3��������Q����ͨ����ⷴӦ�����Ļ�����[H]��ԭij������������õ�����֪QΪNH2OH����Ӧ���ɵ���Ϊ$\frac{5.6L}{22.4L/mol}$=0.25mol������Nԭ���غ��֪Y�в���NԪ�أ�Q�ڼ���������Q�ɽ�Fe��OH��2����ΪFe��OH��3��ͬʱ��������X��X��NԪ�ػ��ϼ۽��ͣ����Ħ������M��X����M��Y����M��Z������֪Y��H2O��XΪNH3��
��� �⣺������һԪ����Q��ǿ���������¿ɷֽ������������X��Y��Z������Z��һ�ִ����к����ܸߵ����壬��ҵ�ϳ��÷���Һ̬��������ã���֪ZΪN2��16.5g��Q����������ʵ�����AgBrǡ�÷�Ӧ����������������Ӧ�����õ�54g����MΪAg�������ʵ���Ϊ$\frac{54g}{108g/mol}$=0.5mol����Q����Է�������Ϊ$\frac{16.5}{0.5}$=33����ϣ�3��������Q����ͨ����ⷴӦ�����Ļ�����[H]��ԭij������������õ�����֪QΪNH2OH����Ӧ���ɵ���Ϊ$\frac{5.6L}{22.4L/mol}$=0.25mol������Nԭ���غ��֪Y�в���NԪ�أ�Q�ڼ���������Q�ɽ�Fe��OH��2����ΪFe��OH��3��ͬʱ��������X��X��NԪ�ػ��ϼ۽��ͣ����Ħ������M��X����M��Y����M��Z������֪Y��H2O��XΪNH3��
��1������Z�ĽṹʽΪN��N������Q�Ļ�ѧʽΪNH2OH��
�ʴ�Ϊ��N��N��NH2OH��
��2����������������NH2OH��Fe��OH��2��Ӧ�����ӷ���ʽΪ��NH2OH+H2O+2Fe��OH��2=NH3��+2Fe��OH��3��
�ʴ�Ϊ��NH2OH+H2O+2Fe��OH��2=NH3��+2Fe��OH��3��
��3������Q����ͨ����ⷴӦ�����Ļ�����[H]��ԭij������������õ������û�����[H]��ԭ�����ã��û�ѧ��Ӧ����ʽΪ��HNO3+6[H]=NH2OH��+2H2O��
�ʴ�Ϊ��HNO3+6[H]=NH2OH��+2H2O��
��4��16.5g����QΪ0.5mol������AgBr��Ӧ����108g Ag��Ag�����ʵ���Ϊ1mol����Ӧ�����N2���⣬�����������ʵ�����AgBr�ķ�Ӧ��ͬ��������HBr��ˮ����NԪ�������������л��ϼ�Ϊa����1mol=0.5mol��[a-��-1��]�����a=2��������N2O���û�ѧ��Ӧ����ʽΪ��2NH2OH+4AgBr=4Ag��+N2O��+4HBr+H2O�����ʵ����������N2Oͬʱ˵��ʵ��ԭ����N2O���ڼ��Է��ӣ�������ˮ������������ֱ�����ˮΪ�ܼ�����Ȫʵ�飬�ܲ�����Ȫ��ΪN2O�����ܲ�����Ȫ��ΪN2��
�ʴ�Ϊ��N2O��2NH2OH+4AgBr=4Ag��+N2O��+4HBr+H2O��N2O���ڼ��Է��ӣ�������ˮ������������ֱ�����ˮΪ�ܼ�����Ȫʵ�飬�ܲ�����Ȫ��ΪN2O�����ܲ�����Ȫ��ΪN2��
���� ���⿼��������ƶϣ���Ŀ�漰���ݲ�����ѧ֪ʶ���������ڲ²���֤�ͣ�ע���ϣ�3���е�����������ѶȽϴ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 25��ʱ��pH=13�İ�ˮ��NaOH��Һ��1.0 L���е�OH-��Ŀ��Ϊ0.1NA | |
| B�� | ���³�ѹ�£���CO2��O2��ɵĻ�����й���NA�����ӣ����е���ԭ����Ϊ2NA | |
| C�� | 22 gD218O�����У��������������������ֱ�Ϊ10NA��12NA | |
| D�� | ��״���£�22.4 L CCl4�к��е�C-Cl����ĿΪ4NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ܢۢݢޢ� | B�� | �٢ݢۢܢޢ� | C�� | �٢ۢܢޢݢ� | D�� | �٢ۢݢޢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
�� ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����� | �۲���¼ | ���ۻ���� |
| A | ��Ũ����ֶ�μ���Cu��ϡ����Ļ��Һ�� | ��������ɫ���� | Ũ����Ļ�ԭ������NO2 |
| B | ��ij�����Һ��pH | pH��7 | NH4+ˮ������NH3•H2O��ʹ��Һ�Լ��� |
| C | ��ʪ��ĵ��۵⻯����ֽ����ij���� | ��ֽ���� | ������һ����Cl2 |
| D | ��20mL pH��Ϊ1������ʹ���ֱ��ˮϡ����pHΪ3 | ���������仯�� | ���������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ù��������������Ʊ���Һʱ���л���Na2O���� | |
| B�� | �ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ | |
| C�� | ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ | |
| D�� | �ζ����յ����ʱ���ּ�ʽ�ζ��ܼ��촦����һ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ۡ���֬�������ʶ��ܷ���ˮ�ⷴӦ����������Ȼ�л��߷��� | |
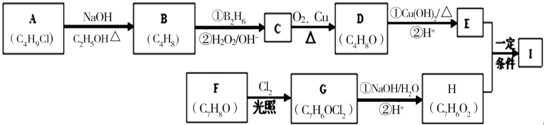
| B�� | �Ҵ���2-������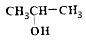 ������ͬϵ���������IJ���Ҳ����ͬϵ��c ������ͬϵ���������IJ���Ҳ����ͬϵ��c | |
| C�� | ���������������������ȫȼ�գ���������������� | |
| D�� | ���ۡ����ء����Ƕ�������Ȼ�߷��ӻ��������ˮ������ж��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
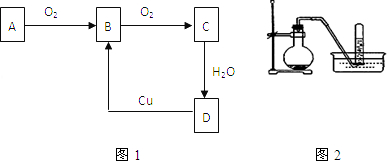
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ˮ������Ӧ��Cl2+H2O?2H++Cl-+ClO- | |
| B�� | �����ƺ�ˮ��Ӧ��Na+2H2O�TNa++2OH-+H2�� | |
| C�� | ���������Ƴ�ȥ�����������Ĥʱ�������ݵķ�Ӧ2Al+2OH-+6H2O�T2[Al��OH��4]-+3H2�� | |
| D�� | ������������ϡ���������ػ����Һ�У������ܽ⣺Fe+2H+�TFe2++H2�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com