


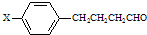 £¬XæÉÄܵĽį¹¹ÓŠ
£¬XæÉÄܵĽį¹¹ÓŠ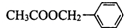 £©ŹĒĘäÖŠµÄŅ»ÖÖ£¬ĖüæÉŅŌ“ÓÜŌĄņ»ØÖŠĢįČ”£¬Ņ²æÉŅŌÓĆ¼×±½ŗĶŅŅ“¼ĪŖŌĮĻ½ųŠŠČĖ¹¤ŗĻ³É£®Ņ»ÖÖŗĻ³ÉĀ·ĻßČē£ŗ
£©ŹĒĘäÖŠµÄŅ»ÖÖ£¬ĖüæÉŅŌ“ÓÜŌĄņ»ØÖŠĢįČ”£¬Ņ²æÉŅŌÓĆ¼×±½ŗĶŅŅ“¼ĪŖŌĮĻ½ųŠŠČĖ¹¤ŗĻ³É£®Ņ»ÖÖŗĻ³ÉĀ·ĻßČē£ŗ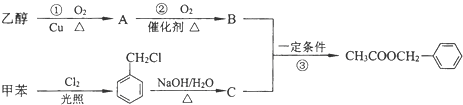


| Cu |
| ”÷ |
| Cu |
| ”÷ |
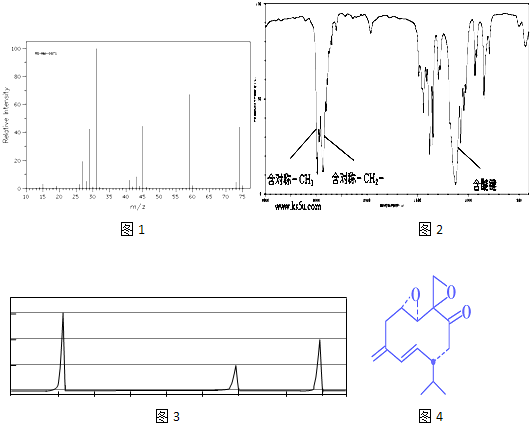
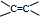 »ņ-C”ŌC-£¬øł¾Żŗ¬ŃõŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ37.21%£¬Ļą¶Ō·Ö×ÓÖŹĮæ²»³¬¹ż100£¬Č·¶Ø·Ö×ÓÖŠŃõŌ×ÓøöŹż£¬½ų¶ųČ·¶ØÓŠ»śĪļµÄĻą¶Ō·Ö×ÓÖŹĮæ£¬Č·¶ØÓŠ»śĪļµÄ·Ö×ÓŹ½£®øł¾ŻŗĖ“Ź²ÕńĒāĘ×Č·¶Ø·Ö×ÓÖŠĒāŌ×ÓĄąŠĶ£¬½įŗĻæÉÄܵĹŁÄÜĶÅ£¬Č·¶ØÓŠ»śĪļ½į¹¹£»
»ņ-C”ŌC-£¬øł¾Żŗ¬ŃõŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ37.21%£¬Ļą¶Ō·Ö×ÓÖŹĮæ²»³¬¹ż100£¬Č·¶Ø·Ö×ÓÖŠŃõŌ×ÓøöŹż£¬½ų¶ųČ·¶ØÓŠ»śĪļµÄĻą¶Ō·Ö×ÓÖŹĮæ£¬Č·¶ØÓŠ»śĪļµÄ·Ö×ÓŹ½£®øł¾ŻŗĖ“Ź²ÕńĒāĘ×Č·¶Ø·Ö×ÓÖŠĒāŌ×ÓĄąŠĶ£¬½įŗĻæÉÄܵĹŁÄÜĶÅ£¬Č·¶ØÓŠ»śĪļ½į¹¹£»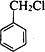 £¬ŌŚ¼īŠŌĢõ¼žĻĀĖ®½āÉś³É
£¬ŌŚ¼īŠŌĢõ¼žĻĀĖ®½āÉś³É £¬
£¬ ÓėCH3COOHŌŚŅ»¶ØĢõ¼žĻĀ·¢Éśõ„»Æ·“Ó¦æÉÉś³É
ÓėCH3COOHŌŚŅ»¶ØĢõ¼žĻĀ·¢Éśõ„»Æ·“Ó¦æÉÉś³É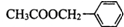 £¬ŅŌ“Ė½ā“šøĆĢā£®
£¬ŅŌ“Ė½ā“šøĆĢā£®| 100”Į37.21% |
| 16 |
| 16”Į2 |
| 37.21% |
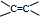 £¬ŌņÓŠ»śĪļĪŖC3H5-COOH£¬ŗĖ“Ź²ÕńĒāĘ×ÓŠ3øö·å£¬ĖµĆ÷ÓŠ3ÖÖĒāŌ×Ó£¬·ūŗĻĢõ¼žµÄ½į¹¹ĪŖ
£¬ŌņÓŠ»śĪļĪŖC3H5-COOH£¬ŗĖ“Ź²ÕńĒāĘ×ÓŠ3øö·å£¬ĖµĆ÷ÓŠ3ÖÖĒāŌ×Ó£¬·ūŗĻĢõ¼žµÄ½į¹¹ĪŖ £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ £»
£»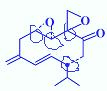 £»ĄūÓĆ²Š»ł·Ø£¬XĪŖ-C5H9O2£¬EÄÜ·¢ÉśŅų¾µ·“Ó¦£¬ĖµĆ÷ÓŠČ©»ł£¬ÄÜÓėĢ¼ĖįĒāÄĘČÜŅŗ·“Ó¦£¬ĖµĆ÷ÓŠōČ»ł£¬ŌņXÖŠÓŠōČ»ł£¬¼“-C4H8COOH£¬
£»ĄūÓĆ²Š»ł·Ø£¬XĪŖ-C5H9O2£¬EÄÜ·¢ÉśŅų¾µ·“Ó¦£¬ĖµĆ÷ÓŠČ©»ł£¬ÄÜÓėĢ¼ĖįĒāÄĘČÜŅŗ·“Ó¦£¬ĖµĆ÷ÓŠōČ»ł£¬ŌņXÖŠÓŠōČ»ł£¬¼“-C4H8COOH£¬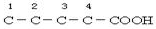 ”¢
Ӣ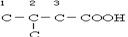 Ӣ
Ӣ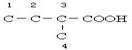 Ӣ
Ӣ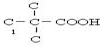 Ӣ
”¢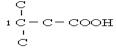 £¬Źż×Ö¶ŌÓ¦µÄĢ¼Ō×ÓÓė±½»·ĻąĮ¬£¬¹²12ÖÖ£¬
£¬Źż×Ö¶ŌÓ¦µÄĢ¼Ō×ÓÓė±½»·ĻąĮ¬£¬¹²12ÖÖ£¬ £¬ŌŚ¼īŠŌĢõ¼žĻĀĖ®½āÉś³É
£¬ŌŚ¼īŠŌĢõ¼žĻĀĖ®½āÉś³É £¬
£¬ ÓėCH3COOHŌŚŅ»¶ØĢõ¼žĻĀ·¢Éśõ„»Æ·“Ó¦æÉÉś³É
ÓėCH3COOHŌŚŅ»¶ØĢõ¼žĻĀ·¢Éśõ„»Æ·“Ó¦æÉÉś³É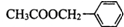 £»
£» £¬
£¬ ŌŚŅ»¶ØĢõ¼žĻĀÓėCH3COOH·¢Éśõ„»Æ·“Ӧɜ³É
ŌŚŅ»¶ØĢõ¼žĻĀÓėCH3COOH·¢Éśõ„»Æ·“Ӧɜ³É £¬·“Ó¦µÄ·½³ĢŹ½
£¬·“Ó¦µÄ·½³ĢŹ½ £¬
£¬ £»õ„»Æ»ņČ”“ś£»
£»õ„»Æ»ņČ”“ś£»| Cu |
| ”÷ |
| Cu |
| ”÷ |
 £¬·“Ó¦¢ŚĪŖ2CH3CHO+O2
£¬·“Ó¦¢ŚĪŖ2CH3CHO+O2| “߻ƼĮ |
| ”÷ |
 £¬¶Ō±ČČżøö·“Ó¦æÉÖŖ·“Ó¦¢ŚŌ×ӵĥķĀŪĄūÓĆĀŹĪŖ100%£¬
£¬¶Ō±ČČżøö·“Ó¦æÉÖŖ·“Ó¦¢ŚŌ×ӵĥķĀŪĄūÓĆĀŹĪŖ100%£¬


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £¬ĻĀĮŠ¶ŌA»ÆѧŠŌÖŹµÄÅŠ¶ĻÖŠ²»ÕżČ·µÄŹĒ£Ø””””£©
£¬ĻĀĮŠ¶ŌA»ÆѧŠŌÖŹµÄÅŠ¶ĻÖŠ²»ÕżČ·µÄŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ijӊ»śĪļAµÄ½į¹¹¼ņŹ½ČēĶ¼£¬ĻĀĮŠÓŠ¹ŲAµÄĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
ijӊ»śĪļAµÄ½į¹¹¼ņŹ½ČēĶ¼£¬ĻĀĮŠÓŠ¹ŲAµÄĖµ·ØÕżČ·µÄŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
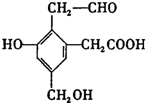 £¬Č”Na”¢NaOH”¢ŠĀÖĘCu£ØOH£©2·Ö±šÓėµČĪļÖŹµÄĮæµÄøĆĪļÖŹĒ”ŗĆ·“Ó¦£Ø·“Ó¦Ź±æɼÓČČÖó·Š£©£¬ŌņNa”¢NaOH”¢ŠĀÖĘCu£ØOH£©2ČżÖÖĪļÖŹµÄĪļÖŹµÄĮæÖ®±ČĪŖ£Ø””””£©
£¬Č”Na”¢NaOH”¢ŠĀÖĘCu£ØOH£©2·Ö±šÓėµČĪļÖŹµÄĮæµÄøĆĪļÖŹĒ”ŗĆ·“Ó¦£Ø·“Ó¦Ź±æɼÓČČÖó·Š£©£¬ŌņNa”¢NaOH”¢ŠĀÖĘCu£ØOH£©2ČżÖÖĪļÖŹµÄĪļÖŹµÄĮæÖ®±ČĪŖ£Ø””””£©| A”¢6£ŗ4£ŗ5 | B”¢1£ŗ1£ŗ1 | C”¢3£ŗ2£ŗ3 | D”¢3£ŗ2£ŗ2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ijӊ»śĪļAµÄ½į¹¹¼ņŹ½ĪŖ£ŗ
ijӊ»śĪļAµÄ½į¹¹¼ņŹ½ĪŖ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
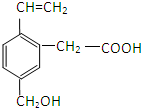 £¬ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
£¬ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ£Ø””””£©| A”¢1mol AÄÜøś2mol NaOHČÜŅŗ·“Ó¦ | B”¢ÄÜ·¢Éś¼Ó¾Ū·“Ó¦ | C”¢ÄÜ·¢Éś·Ö×ÓÄŚõ„»Æ·“Ó¦ | D”¢A·Ö×ÓÖŠĖłÓŠŌ×ÓŌŚĶ¬Ņ»Ę½ĆęÉĻ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com