
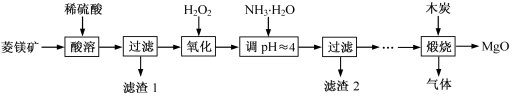
 2MgO+2SO2��+CO2��
2MgO+2SO2��+CO2�� MgO+SO2��+CO��
MgO+SO2��+CO�� MgO+S��+3CO��
MgO+S��+3CO��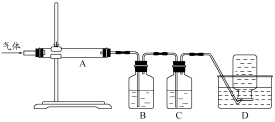
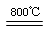
 2S2��+SO32��+3H2O (2��)
2S2��+SO32��+3H2O (2��) 2S2��+SO32��+3H2O��������CO ��d ��3S+6OH��
2S2��+SO32��+3H2O��������CO ��d ��3S+6OH�� 2S2��+SO32��+3H2O��
2S2��+SO32��+3H2O��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ���� | ����ʽ | �۵�/�� | �е�/�� | �ܶ�/g��cm-1 | �ܽ��� |
| �Ҷ��� | C2H4O2 | ��11.5 | 198 | 1.11 | ������ˮ���Ҵ� |
| ������ | C3H8O3 | 17.9 | 290 | 1.26 | �ܸ�ˮ���ƾ�������Ȼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������Ȼ�̼ | B�������屽 | C�����ͺͱ� | D����������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����̢���Ҫ���뻯ѧ�Լ������������˵Ȳ��� |
| B���ɡ�ĸҺ����ˮMgCl2��һϵ�б仯��δ�漰������ԭ��Ӧ |
| C����ҵ��һ���ý���������ˮMgCl2��Ӧ��ȡMg���� |
| D����Ӧ�ۺ͢ݾ��������з�Ӧʵ�֣�2Br-+Cl2=Br2+2Cl-���÷�Ӧ�����û���Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������ȥ���������л������������� |
| B����Na2CO3��Һ��ȥ������̼�������Ȼ��� |
| C��������ķ�����ȥˮ�������������ȡ����ˮ |
| D���÷�Һ�ķ����������CCl4�Ļ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| | NaNO3 | KNO3 | NaCl | KCl |
| 10�� | 80.5 | 21.2 | 35.7 | 31.0 |
| 100�� | 175 | 246 | 39.1 | 56.6 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
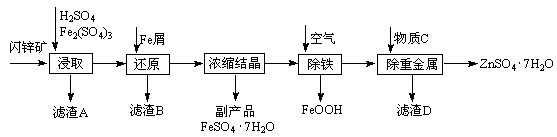
| �¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
| �ܽ��/g | 41.8 | 54.1 | 70.4 | 74.8 | 67.2 | 60.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
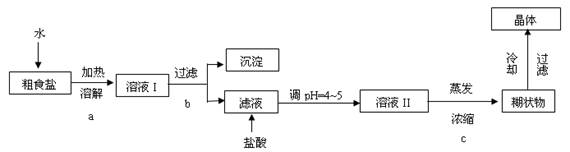
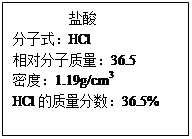
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com