
ij����Ͷ�ʽ���һ����ҵ�ƾ�����Ŀ�����ù�ҵ�ƾ������ͻ���Ƴɡ��Ҵ����͡����Խ�ʡʯ����Դ����֪�ƾƾ��ķ��������֣�
���ڴ�����������ϩ��ˮ��Ӧ��
��CH3CH2Br��H2O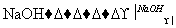 CH3CH2OH��HBr��
CH3CH2OH��HBr��
��(C6H10O5)n(����)��nH2O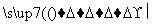 nC6H12O6(������)��
nC6H12O6(������)��
C6H12O6(������)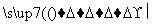 2C2H5OH��2CO2����
2C2H5OH��2CO2����
(1)�����ٵĻ�ѧ����ʽ��________________________________________��
(2)�����ڵĻ�ѧ��Ӧ������____________��
(3)Ϊ����ʯ�Ͷ�ȱ��������ԴΣ��������Ϊ����Ӧѡ����һ�ַ���������ҵ�ƾ������������
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
(4)�������ɫ��ѧ(��ԭ�������ʡ����)�ĽǶȿ����ƾƾ���õ�һ�鷽����________��
A���� B����
C���٢� D���٢ڢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
A��ԭ����������������3(С��8)��Ԫ��һ���Ƿǽ���Ԫ��
B��ԭ�������ֻ��1�����ӵ�Ԫ��һ���ǽ���Ԫ��
C�������������ȴ������������Ԫ��һ��λ�ڵ�2����
D��ijԪ�ص����ӵ�������������������ͬ����Ԫ��һ��λ�ڵ�3����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����AlCl3��MgSO4�����Һ�������в��ϼ���NaOH��Һ���õ��ij������������NaOH��Һ�������ͼ��ʾ����ԭ��Һ��Cl����SO �����ʵ���֮��Ϊ(����)
�����ʵ���֮��Ϊ(����)
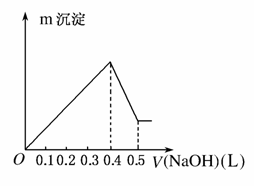
A��1:1 B��2:3
C��3:2 D��6:1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ҫ�ɷ��Ǵ��ᣬ�����ճ��������кܶ����á�
(1)����ʱ��������ʳ���Ͼƿ���ʹ���Ƶ�������������ζ��������ζ������________��
A��ʳ��
B��ʳ���е�����
C���Ͼ��е��Ҵ�
D���Ͼ��е��Ҵ���ʳ���е����ᷴӦ���ɵ���������
(2)��ˮ���þ��˻����ڱڲ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ���ڼ���ʱ����е���״ף����ɳ�ȥˮ����д����һ�����з�����Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڵ��۵�˵���в���ȷ����(����)
A������������ˮ
B���������ڸ߷��ӻ�����
C���������ˮ���ó�����ɫ
D���������������ܹ�ˮ�⣬���������ǣ����������ս���ѪҺ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У����γ��������(����)
A���������� B�����ȴ���
C��������̼ D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����6��̼ԭ�ӵ������������ѻ������ɵ����������(����)
A��3�� B��4��
C��5�� D��6��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ����Ҫ�ù����Ȼ���ȷ����0.5 L 0.2 mol��L��1��NaCl��Һ�����������������DZ���ʹ�õ�(����)
A���Թ� B����ͷ�ι�
C��500 mL������ƿ D��������ƽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ��a��b������ʯī��������������ȷ����(����)
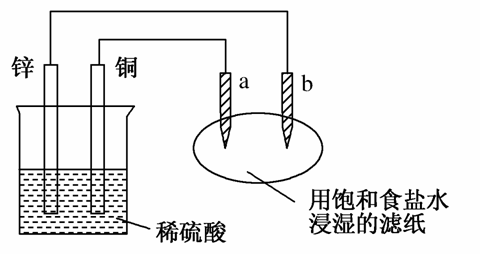
A��a��������������ԭ��Ӧ
B��b������������������Ӧ
C��ϡ��������������ӵ����ʵ�������
D������ֽ�ϵμӷ�̪��Һ��a��������ɫ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com