
£Ø1£©ČÜŅŗÖŠc(H+)Ōö“óµÄŹĒ_________________________________________£»
£Ø2£©ČÜŅŗÖŠpHŌö“óµÄŹĒ___________________________________________£»
£Ø3£©ČÜŅŗÖŠc(H+)»ł±¾²»±äµÄŹĒ______________________________________£»
£Ø4£©Īö³öĘųĢå×ÜĢå»ż×ī“óµÄŹĒ_______________________________________£»
£Ø5£©Ņõ¼«Īö³ö½šŹōÖŹĮæ×ī“óµÄŹĒ_____________________________________”£
£Ø1£©¢Ł¢Ż (2)¢Ū¢Ü (3)¢Ś¢Ž (4)¢Ü (5)¢Ł
½āĪö£ŗÓƶčŠŌµē¼«µē½āĖ®ČÜŅŗµÄø÷ÖÖĒéæöČēĻĀ
ĄąŠĶ | µē¼«·“Ó¦ĢŲµć | Ąż×Ó | Źµ¼Źµē½ā¶ŌĻó | µē½āÖŹÅØ¶Č | ČÜŅŗpH | µē½āÖŹČÜŅŗø“Ō |
µē½āĖ®ŠĶ | Ńō¼«ĒāŃõøł·Åµē Ņõ¼«ĒāĄė×ӷŵē | NaOH | H2O | Ōö“ó | Ōö“ó | ¼ÓH2O |
H2SO4 | H2O | Ōö“ó | ¼õÉŁ | ¼ÓH2O | ||
Na2SO4 KNO3 | H2O | Ōö“ó | ²»±ä | ¼ÓH2O | ||
µē½āÖŹ ·Ö½āŠĶ | µē½āÖŹµÄĄė×ӷŵē | HCl CuCl2 | HCl CuCl2 | ¼õÉŁ¼õÉŁ | Ōö“óŌö“ó | ¼ÓHCl ¼ÓCuCl2 |
·Å³öĒāĘų Éś³É¼īŠĶ | Ńō¼«ĒāĄė×ӷŵē | NaCl KBr | µē½āÖŹŗĶĖ® | ²æ·ÖĄė×Ó ÅØ¶Č¼õÉŁ | Ōö“ó | ¼ÓHCl ¼ÓHBr |
·Å³öŃõĘų Éś³ÉĖįŠĶ | Ńō¼«ĒāŃõøł·Åµē | CuSO4 AgNO3 | µē½āÖŹŗĶĖ® | ²æ·ÖĄė×Ó ÅØ¶Č¼õÉŁ | ¼õÉŁ | ¼ÓCuO¼ÓAg2O |


 ѧĮ·æģ³µµĄæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
ѧĮ·æģ³µµĄæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā


| ŹµŃé·½·Ø | ŹµŃéĻÖĻó | ½įĀŪ |
| ŌŚČÜŅŗÖŠ¼Ó KSCNČÜŅŗ |
ČÜŅŗĪŖŗģÉ« ČÜŅŗĪŖŗģÉ« |
¹ĢĢåĪļÖŹÖŠÓŠFeCl3 |
| ĻņaČÜŅŗÖŠµĪ¼Ó ĖįŠŌKMnO4ČÜŅŗ |
KMnO4ČÜŅŗ×ĻÉ« ²»ĶŹÉ« |
¹ĢĢåĪļÖŹÖŠ²»ŗ¬ ¹ĢĢåĪļÖŹÖŠ²»ŗ¬ FeCl2 ¹ĢĢåĪļÖŹÖŠ²»ŗ¬ FeCl2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠĻĀĮŠµē½ā×°ÖĆ£ŗ¢ŁÓĆ²¬µē¼«µē½āAgNO3ČÜŅŗ ¢ŚÓĆŠæµē¼«µē½āZnCl2ČÜŅŗ ¢ŪÓĆŹÆÄ«µē¼«µē½āNaOHČÜŅŗ ¢ÜÓĆ²¬µē¼«µē½āNaClČÜŅŗ ¢ŻÓĆ²¬µē¼«µē½āCuSO4ČÜŅŗ ¢ŽÓĆ²¬µē¼«µē½āNa2SO4ČÜŅŗ”£µ±ŌŚĻąĶ¬µÄŹ±¼äÄŚĶØĻąĶ¬Ēæ¶ČµÄµēĮ÷Ź±£ŗ£ØÓĆŠņŗÅĢīŠ“£©
£Ø1£©ČÜŅŗÖŠc(H+)Ōö“óµÄŹĒ_________________________________________£»
£Ø2£©ČÜŅŗÖŠpHŌö“óµÄŹĒ___________________________________________£»
£Ø3£©ČÜŅŗÖŠc(H+)»ł±¾²»±äµÄŹĒ______________________________________£»
£Ø4£©Īö³öĘųĢå×ÜĢå»ż×ī“óµÄŹĒ_______________________________________£»
£Ø5£©Ņõ¼«Īö³ö½šŹōÖŹĮæ×ī“óµÄŹĒ_____________________________________”£
²éæ““š°øŗĶ½āĪö>>
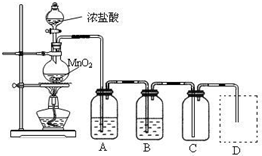
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
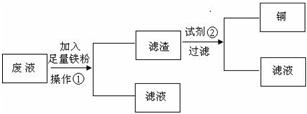
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗÕć½Ź”Ä£ÄāĢā ĢāŠĶ£ŗŹµŃéĢā





²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com