
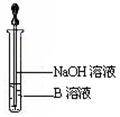
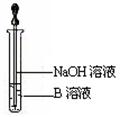
��14�֣�ijͬѧ������ͼװ���Ʊ���������������a�Թ�����Ũ���ᡢ�Ҵ�������Ļ��Һ�� b�Թ�ʢ����Na2CO3��Һ���ش��������⣺
��1��ָ��װ����һ�����ԵĴ���
��2������Ũ���ᡢ�Ҵ�������Ļ��Һ����ȷ����˳���� ��
��3��д���Թ�a����������Ӧ�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��
��4����Ӧ��������b�ԹܵĻ��Һ���ɹ۲쵽b�Թ�����ϸС����ð����д����ʾ�÷�Ӧ�����ӷ���ʽ ��
��5����b�Թ��з�������������ķ��뷽���� ��
��6��Ũ�����ڴ˷�Ӧ�е������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ��������ˮһ�и�һ��ѧ����ĩģ�⻯ѧ�Ծ����������� ���ͣ�ʵ����
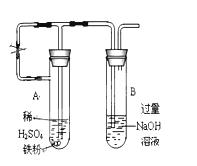
(17��)ijͬѧ������ͼ��ʾ��ʵ��װ�ý�������ˮ������Ӧ��ʵ��,�������о������仯����IJ������ʡ�
��֪:��FeO + 2H+ = Fe2+ + H2O��Fe2O3 + 6H+ = 2Fe3+ +3 H2O ��Fe3O4 + 8H+ = Fe2+ +2Fe3+ +4 H2O
��ش���������:
��1��Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����ͬѧ��ȷ����Ӧһ��ʱ���Ӳ���Թ��й������ʵijɷ�,���������ʵ�鷽��:
�ٴ�Ӳ���Թ���ȴ��,ȡ�������еĹ�����������ϡ�������ҺB;
��ȡ������ҺB�μ�KSCN��Һ,����Һ���ɫ��˵��Ӳ���Թ��й������ʵijɷ���(ֻ��һ��ѡ���������) ,����Һδ���ɫ��˵��Ӳ���Թ��й������ʵijɷ���(ֻ��һ��ѡ���������) ��
| A��һ����Fe3O4,������Fe | B��ֻ��Fe(OH)3 | C��һ����Fe3O4��Fe |
| D��һ����Fe(OH)3,������Fe E.ֻ��Fe3O4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ɽ��ʡ�����и�һ��ѧ����ĩģ�⻯ѧ�Ծ��������棩 ���ͣ�ʵ����
(17��)ijͬѧ������ͼ��ʾ��ʵ��װ�ý�������ˮ������Ӧ��ʵ��,�������о������仯����IJ������ʡ�
��֪:��FeO + 2H+ = Fe2+ + H2O��Fe2O3 + 6H+ = 2Fe3+ +3 H2O ��Fe3O4 + 8H+ = Fe2+ +2Fe3+ +4 H2O
��ش���������:
��1��Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����ͬѧ��ȷ����Ӧһ��ʱ���Ӳ���Թ��й������ʵijɷ�,���������ʵ�鷽��:
�ٴ�Ӳ���Թ���ȴ��,ȡ�������еĹ�����������ϡ�������ҺB;
��ȡ������ҺB�μ�KSCN��Һ,����Һ���ɫ��˵��Ӳ���Թ��й������ʵijɷ���(ֻ��һ��ѡ���������) ,����Һδ���ɫ��˵��Ӳ���Թ��й������ʵijɷ���(ֻ��һ��ѡ���������) ��
A��һ����Fe3O4,������Fe B��ֻ��Fe(OH)3 C��һ����Fe3O4��Fe
D��һ����Fe(OH)3,������Fe E.ֻ��Fe3O4
��3����ͬѧ������ʵ�鷽��������ʵ��,�����Һδ���ɫ,ԭ����
�������ӷ���ʽ��ʾ����
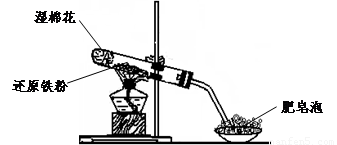
��4����ͬѧ������ȡ������ҺB,ʹ���NaOH��Һ��Ӧ��������ͼ��ʾ�IJ���,�ɹ۲쵽���ɰ�ɫ����,Ѹ�ٱ�ɻ���ɫ,����ɺ��ɫ������,��д��������������صķ�Ӧ�Ļ�ѧ����ʽ ��
��5��һ��ʱ���,��ͬѧ���֣�3����δ������Һ��ɺ�ɫ,˵��Fe2+ ���� �ԡ��ɴ˿�֪,ʵ�����к�Fe2+������Һ�����������Ƶ�ԭ���� ���������ƺ�Fe2+������ҺʱӦ�������� ��
��6����ͬѧΪ�˻�ó־ð�ɫ��Fe(OH)2������������ͼ��ʾװ�ã��ò���O2������ˮ���Ƶ�NaOH��Һ�����Ƶ�FeSO4��Һ��Ӧ����ò���O2������ˮ�ķ�����______________����Ӧ��ʼʱ����ֹˮ�е�Ŀ����___________________________________��һ��ʱ��ر�ֹˮ�У����Թ�_______���A����B�����й۲쵽��ɫ��Fe(OH)2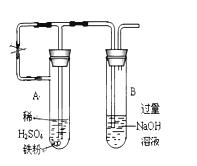
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ɽ��ʡ�����и����ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ʵ����
��15�֣��ߴ�������������Fe2O3�����ִ����ӹ�ҵ����Ҫ���ϡ�ʵ��������������������Ҫ�ɷ�ΪFe2O3��FeO��������SiO2�����ʣ�Ϊԭ���Ʊ��ߴ��������IJ������£�

�ش��������⣺
 ��1������ʵ�����漰�ķ�Ӧ�У���һ����Ӧ�����ڻ��Ϸ�Ӧ��������������ԭ��Ӧ��д���÷�Ӧ�����ӷ���ʽ��
��
��1������ʵ�����漰�ķ�Ӧ�У���һ����Ӧ�����ڻ��Ϸ�Ӧ��������������ԭ��Ӧ��д���÷�Ӧ�����ӷ���ʽ��
��
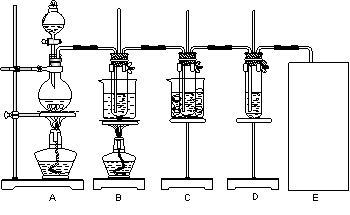
��2��ʵ��������18.4mol��L-1��Ũ��������100mL 5.0mol��L-1������
��Һ�����õIJ���������ͷ�ιܡ���Ͳ���ձ����������⣬����
����д�������ƣ���
��3��ijͬѧ����ͼ��ʾװ�ý��й��˲�����
����ָ�����еĴ���֮���� ��
�ڹ��˺�ϴ�ӹ����������������ķ����� ��
��4��ijͬѧ����ͼ��ʾװ�ã�β������װ��δ������ʵ������ҺY��ͨ��NH3��CO2

������Ϊʵ�����Ʊ�NH3��CO2�ı�ѡҩƷ��
a.NH4Cl b.CaCO3����״�� c.Ca��OH��2 d.NaOH
e.Ũ��ˮ f.ϡ���� g.ϡ����
������װ��A�����Թ�������ҩƷ�����ѡ��Ϊ �� ����ҩƷ�����գ���װ��D��ҩƷ�����ѡ��Ϊ �� ����ҩƷ�����գ���
�����и����Ʊ�ʵ���У�Ҳ������װ��D��������ɵ��� ������ţ���
A��MnO2��Ũ���ᷴӦ�Ʊ�Cl2
B��Cu��Ũ���ᷴӦ����SO2
C����KMnO4�ֽ���O2
D���Ҵ������ᷴӦ�Ʊ���������
E��Zn��ϡ���ᷴӦ�Ʊ�H2
��д������װ��A�����Թ�����������Ӧ�Ļ�ѧ����ʽ ��
����ͨ��һ������NH3��CO2��װ��C������Һ��ֻ����S��N��H��O����Ԫ�ء���pH��ֽ�ⶨ����ҺpH�ķ����� ��������Һ�����ԣ�����Һ�е�NH+4��SO2-4�����ʵ���Ũ�ȼ��������ϵΪ �������ӵ�Ũ���÷���[NH+4]��[SO2-4]��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ʵ�������������;ʮ�ֹ㷺�����������Ʊ����塣
��1��ijС��ͬѧ����4 mol/L����������230 mL 0.4mol/L ������Һ����ȡ��4 mol/L������ ���� ������������mL��ʵ������Ҫ�õ��IJ��������������� ���� �������������� ����Ͳ������ �ձ�����ͷ�ιܡ�
��2������ʵ�����ʹ���Ƶ���ҺŨ��ƫ�͵���������������
���� A������ƿϴ�Ӻ�δ����
���� B����Һʱ����������Һ����
���� C������ʱ����ˮ���������̶��ߣ��ֵ���һЩ
���� D������ʱ�����ӿ̶���
���� E��װ���Լ�ƿʱ����������Һ����
����16�֣���仯ѧ�����ս����̿���Ҫ�ɷ�ΪMnO2����Ũ�����ϼ��ȣ������������ȵõ�����������֪Cl2�ͼ���Һ�ڲ�ͬ�����£��õ��IJ��ﲻͬ��ij��ȤС������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ��� 3Cl2+6KOH����![]() KClO3+5KCl+3H2O ��
KClO3+5KCl+3H2O ��
 |
����
�� ͼ�У�AΪ��������װ�ã�B���Թ���ʢ��15 mL 30% KOH��Һ��������ˮԡ�У�C���Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У�D���Թ��������ɫʯ����Һ��
����д���пհף�
��1����ȡ����ʱ����Բ����ƿ�����һ�������Ķ������̣�ͨ������������ (����������)��Բ����ƿ�м���������Ũ���ᡣװ��A�з�Ӧ�����ӷ���ʽ�������� ��������������������
��2��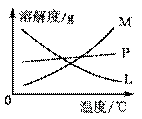 ��Ӧ��Ͼ���ȴ��B���Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ��������� (������ĸ)����B���Թ��з�����þ���ķ������������� (��ʵ���������)����С��ͬѧ�����Ƶõ�����ز���ƫ�ͣ����ܵ�һ��ԭ����Cl2�к������ʣ������ʳɷ��������� ����ˮ�����⣬�ѧʽ��,��θĽ��������������������������������������������������������������� ��
��Ӧ��Ͼ���ȴ��B���Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ��������� (������ĸ)����B���Թ��з�����þ���ķ������������� (��ʵ���������)����С��ͬѧ�����Ƶõ�����ز���ƫ�ͣ����ܵ�һ��ԭ����Cl2�к������ʣ������ʳɷ��������� ����ˮ�����⣬�ѧʽ��,��θĽ��������������������������������������������������������������� ��
��3��ʵ���пɹ۲쵽D���Թ�����Һ����ɫ���������±仯������д�±��еĿհף�
| ʵ������ | ԭ�� |
| ��Һ�������ɫ��Ϊ���� ɫ | �� ������ˮ��Ӧ���ɵ�H��ʹʯ���ɫ |
| �����Һ��Ϊ��ɫ | �� ������������������������������������������ |
��4������װ��ͼ�����л���ȱ�ٵ�ʵ��װ�ã���ע���Լ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡ�����и����ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ʵ����
��15�֣��ߴ�������������Fe2O3�����ִ����ӹ�ҵ����Ҫ���ϡ�ʵ��������������������Ҫ�ɷ�ΪFe2O3��FeO��������SiO2�����ʣ�Ϊԭ���Ʊ��ߴ��������IJ������£�
�ش��������⣺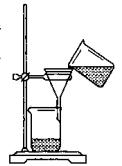 ��1������ʵ�����漰�ķ�Ӧ�У���һ����Ӧ�����ڻ��Ϸ�Ӧ��������������ԭ��Ӧ��д���÷�Ӧ�����ӷ���ʽ�� ��
��1������ʵ�����漰�ķ�Ӧ�У���һ����Ӧ�����ڻ��Ϸ�Ӧ��������������ԭ��Ӧ��д���÷�Ӧ�����ӷ���ʽ�� ��
��2��ʵ��������18.4mol��L-1��Ũ��������100mL 5.0mol��L-1������
��Һ�����õIJ���������ͷ�ιܡ���Ͳ���ձ����������⣬����
����д�������ƣ���
��3��ijͬѧ����ͼ��ʾװ�ý��й��˲�����
����ָ�����еĴ���֮���� ��
�ڹ��˺�ϴ�ӹ����������������ķ����� ��
��4��ijͬѧ����ͼ��ʾװ�ã�β������װ��δ������ʵ������ҺY��ͨ��NH3��CO2
������Ϊʵ�����Ʊ�NH3��CO2�ı�ѡҩƷ��
a.NH4Cl b.CaCO3����״�� c.Ca��OH��2 d.NaOH
e.Ũ��ˮ f.ϡ���� g.ϡ����
������װ��A�����Թ�������ҩƷ�����ѡ��Ϊ �� ����ҩƷ�����գ���װ��D��ҩƷ�����ѡ��Ϊ �� ����ҩƷ�����գ���
�����и����Ʊ�ʵ���У�Ҳ������װ��D��������ɵ��� ������ţ���
| A��MnO2��Ũ���ᷴӦ�Ʊ�Cl2 |
| B��Cu��Ũ���ᷴӦ����SO2 |
| C����KMnO4�ֽ���O2 |
| D���Ҵ������ᷴӦ�Ʊ��������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com